A review on genesis of authigenic carbonate fluorapatite in marine sediments
-
摘要:
自生碳氟磷灰石(CFA)是海洋沉积物中重要的磷汇,也是海底沉积型磷矿的主要含磷矿物。解析CFA的成因对了解地质历史时期海洋生产力变化、磷循环模式及其全球气候环境效应等具有重要的科学意义。本文在比较全面地收集、整理已有海洋沉积物中自生碳氟磷灰石成因研究相关文献和资料的基础上,通过综合性的比较分析,全面地总结了有关海洋沉积物中CFA形成的物质来源、形成环境及沉淀机制的认识,分析了包括有机质的微生物降解、铁羟基氧化物对磷酸盐的吸附与释放、鱼类硬质碎屑的溶解、大型硫化细菌对多聚磷酸盐的储存与释放等有关磷富集的过程,揭示了氧化还原条件的波动等对磷富集的影响。同时,本文强调磷酸钙(CaP)前体的存在及与CFA形成之间可能的关系,阐释CaP前体在碳酸钙表面的界面耦合溶解-沉淀机制可作为CFA交代成因的微观证据,并明确了交代成因CFA的多种鉴别标志。最后,希望依靠海洋科技的进步以及多学科的交叉研究,提出未来进一步深入研究海洋沉积物中自生CFA成因与分布的重要方向。
Abstract:Authigenic carbon fluorapatite (CFA) is a crucial phosphorus sink in marine sediments and is the primary phosphorus-bearing mineral in submarine phosphorites. Understanding the genesis of CFA is of great scientific significance for understanding the changes in marine productivity, phosphorus cycling, and global climate and environmental effects throughout geological history. We overviewed the material sources, formation environment, and precipitation mechanisms of CFA in marine sediments. The enrichment of phosphorus in porewater involves the microbial decomposition of organic matter, the adsorption and release of phosphate by ferric oxyhydroxides, and the storage and utilization of polyphosphates by large sulfide bacteria. Fluctuations in redox conditions exert a significant influence on these processes. The formation of calcium phosphate (CaP) precursor phase is an important pathway for CFA precipitation. Moreover, the interface coupled dissolution and precipitation (ICDP) mechanism of CaP on calcium carbonate surfaces reveals the alteration genesis of CFA from a microscopic perspective. Based on these findings, future research directions for investigating the genesis of authigenic CFA in marine sediments are also proposed.
-
黄河三角洲北部河口地区位于山东省东北部,区内软土分布广泛,主要为新近沉积黏土、淤泥质粉质黏土以及淤泥。黄河三角洲研究程度较高[1-16],但对河口区软土这类不良土体的工程特性、空间分布规律等尚未进行过系统研究。随着黄河三角洲地区上升为国家战略,以及国家生态文明建设的要求,基于各类工程建设基础稳定性要求,对在该地区分布广泛、厚度较大的不良土体软土工程特性及分布的研究显得尤其重要。鉴于此,本文利用近年来在该地区开展的国家基金及省地勘项目成果,运用最新的大量工程地质钻孔实测数据资料,以现代数理统计分析为手段,对软土的物理力学性质、空间分布、沉积环境及物质成分等方面进行了综合分析,给出该区软土工程特性及分布规律。本文结果对于该地区的生态文明建设及经济可持续发展具有支持价值。
1. 研究区概况
黄河三角洲是一典型扇形三角洲(图1),由黄河携带泥沙不断淤积扩张填海造陆形成,属河流冲积物覆盖海相层的二元相结构[17-18]。黄河三角洲地区受黄河下游冲淤积及滨海沉积作用,地层岩性较为复杂,存在较多沉积相变、间断及透镜体格局,沉积模式主要与河流过程、黄河来水、泥沙有关。在这种海相沉积层和河口三角洲相沉积层不断沉积变化过程中,有较多软土层分布,这种含水量大、压缩性高、承载力低的软土是黄河三角洲及河口地区重要的地质环境问题之一。
河口区地处现代黄河三角洲平原北部,该区濒临渤海,是河流的最下游。河道游荡较频繁,地形地貌反复变化,水沙条件也随之变化,因此,区内土体结构一般无巨厚和广延的单层岩性沉积,多为多层沉积结构[19]。在垂向上,岩性沉积为河流沉积陆相层夹薄层海相层沉积,层理较为发育,大小互层叠置是本区沉积结构上的重要特征。本文依据在黄河三角洲河口地区的92个工程地质钻探孔资料,分析总结该区软土工程特性及分布规律。
2. 软土工程地质特性及分布
2.1 物理力学性质特征
软土类土一般是指在水流缓慢的沉积环境中和有微生物参与作用的条件下沉积形成的含较多有机质、疏松软弱(天然孔隙比大于1,天然含水率大于液限)的含较多粉粒的黏性土。它一般是近代未经固结的在滨海、湖泊、沼泽、废河道等地区沉积的一种特殊土类[20-24],包括淤泥、淤泥质土、淤泥质粉质黏土等,由于形成条件近似,不同成因形成的软土类土的性质是很相似的。黄河三角洲地区地处渤海之滨,具有软土的沉积环境,根据本次黄河三角洲北部1∶5万综合水工环地质调查中原状测试样品的物理力学指标的统计,不论浅海相、过渡相黏性土,还是三角洲相黏性土,软土主要物理力学特征表现为天然含水量高,绝大多数大于35%,天然含水量大于液限,饱和度高,高孔隙比(大于1.0),透水性极弱,高压缩性(图2),多呈软塑或流塑状态,具较显著的触变性和蠕变性,抗剪强度和地基承载力低,地基承载力普遍小于90 kPa。表1为92个实际钻孔的152件软土样品的测试指标统计值。
表 1 黄河三角洲北部(河口区)软土的物理力学性质指标统计值Table 1. Physical and mechanical properties of soft soil in the study area指标 范围值 平均值 指标 范围值 平均值 含水率w/% 32.6~60.2 43.55 塑限wp/% 18.1~29.1 21.91 比重Gs 2.71~2.76 2.74 塑性指数Ip 11.1~25.8 16.85 密度ρ/(g.cm−3) 1.62~1.88 1.79 液性指数IL 1.00~1.65 1.29 干密度ρd/(g.cm−3) 1.02~1.36 1.25 压缩系数α/MPa 0.43~1.39 0.77 孔隙比e 1.005~1.713 1.21 压缩模量Es/MPa 1.78~4.86 3.05 饱和度Sr/% 88~100 97.73 黏聚力c/kPa 11.1~28.2 16.88 液限wL/% 29.6~54.9 38.76 内摩擦角φ/(°) 0.5~8.3 3.69 2.1.1 物理力学性质指标概率特征
在样品的钻探采集过程中,由于人为、机器等不确定因素都会引起样品一系列测值的变异性,以致软土参数很难被准确确定,研究成果的代表性不足,因此,准确地分析掌握软土参数的统计概率分布,对于软土工程特性研究的准确性至关重要[25-26]。本文通过对该地区最新钻探钻孔软土样品的土工试验分析数据,对黄河三角洲北部(河口区)软土(淤泥质土、淤泥质粉质黏土和淤泥)样品的各项物理力学性质指标的概率分布分别进行数理统计分析,从主要指标的概率分布图(图3)中可以看出,河口地区软土的含水量、孔隙比、液限、塑限、压缩系数、压缩模量基本符合正态分布,这也提高了本文在该地区开展软土特性研究数据的准确性和可靠度。通过计算,软土样品物理性质指标中,比重、密度、含水率、孔隙比、液限、塑限变异系数δ分别为0.001、0.03、0.14、0.14、0.13、0.10,均小于0.2,表现了较好的稳定性;力学性质各项指标中压缩系数、压缩模量变异系数δ分别为0.28、0.22,稳定性相对较好。
2.1.2 物理力学性质指标相关性特征
从图4中可见,黄河三角洲北部河口地区软土随着液限的增大,其塑性指数整体呈增大态势,液限与塑性指数呈显著线性相关,相关系数R2达到0.98。在我国塑性图(图5)上,研究区域的软土数据大都落在A线以上、C线的右侧,靠近A线并大致平行于A线分布,为塑性较高的淤泥质粉质黏土、淤泥质黏土[7, 28]。
从图6可以看出,黄河三角洲北部地区软土测试指标中的含水量与孔隙比呈现线型正相关关系,孔隙比随着含水量的不断增加而增加,相关系数R2达到0.93,相关性非常显著。同理,由图7可以看出,该地区软土孔隙比与压缩系数呈现线性相关性关系,压缩系数随着孔隙比不断增长亦随之增长,相关系数R2达到0. 62,相关性较高。
2.2 软土空间分布特征
研究依据92个实际钻孔的土工试验成果表中天然含水率、液限、土命名等物理力学指标数据来判定黄河三角洲北部(河口区)的软土分布区域。钻探结果表明,区内软土呈片状分布,并呈现大面积广泛分布,软土分布面积约占总面积的85%,北部沿海地段皆为软土分布区域。从钻探岩心编录及取样分析来看,该区域内软土厚度一般不超过15 m,最小厚度1 m左右,最大厚度11 m左右且位于仙河镇四号桩附近(表2),平面分布和厚度不均匀(图8),从垂向空间上看,软土层厚度大多数小于5 m,顶板埋深大多小于5 m或5~10 m,尤其是在滨海海岸带滩涂近海区域,顶板埋深较浅,大于10 m的局部分布,底板埋深多数为10~20 m(图9)。
表 2 黄河三角洲北部(河口区)软土分布钻孔及特征Table 2. Distribution and characteristics of drilling holes in the north of Yellow River delta(Estuarine district)钻孔
编号软土
分布软土命名 顶板埋
深/m软土总
厚度/m钻孔
编号软土
分布软土命名 顶板埋
深/m软土总
厚度/mZT01 有 淤泥质粉质黏土 8.0 4.0 ZT45 有 淤泥质粉质黏土、淤泥质黏土 10.6 5.1 ZT02 有 淤泥质粉质黏土 7.9 1.3 ZT46 有 淤泥质粉质黏土、淤泥质黏土 7.0 9.0 ZT03 有 淤泥质粉质黏土 14.0 2.7 ZT47 有 淤泥质粉质黏土、淤泥质黏土 7.0 10.8 ZT05 有 淤泥质黏土 8.3 1.4 ZT48 有 淤泥质粉质黏土 2.1 6.5 ZT06 有 淤泥质粉质黏土、淤泥质黏土、淤泥 7.3 6.0 ZT50 有 淤泥质粉质黏土 4.5 8.5 ZT07 有 淤泥质黏土 5.0 8.9 ZT51 有 淤泥、、淤泥质黏土、淤泥质粉质黏土 4.0 6.0 ZT08 有 淤泥质黏土 10.0 2.3 ZT52 有 淤泥质粉质黏土、淤泥质黏土 4.0 8.8 ZT10 有 淤泥质粉质黏土 9.5 3.8 ZT56 有 淤泥质粉质黏土 7.0 8.0 ZT11 有 淤泥质粉质黏土 6.0 6.7 ZT57 有 淤泥质粉质黏土 2.8 1.2 ZT12 有 淤泥质黏土 11.8 2.2 ZT58 有 淤泥质黏土 10.7 1.3 ZT13 有 淤泥质粉质黏土、淤泥质黏土 11.5 6.2 ZT59 有 淤泥质黏土、淤泥质粉质黏土 6.5 5.0 ZT15 有 淤泥质粉质黏土 14.0 2.0 ZT60 有 淤泥质黏土 11.2 2.7 ZT16 有 淤泥质粉质黏土、淤泥质黏土 7.7 2.6 ZT61 有 淤泥质粉质黏土 0.0 2.8 ZT17 有 淤泥质粉质黏土 7.0 2.0 ZT63 有 淤泥质粉质黏土 7.9 1.5 ZT18 有 淤泥质粉质黏土 9.0 1.0 ZT64 有 淤泥质粉质黏土 5.6 1.7 ZT20 有 淤泥质粉质黏土 16.0 1.9 ZT65 有 淤泥、淤泥质黏土 2.4 6.1 ZT21 有 淤泥质粉质黏土 10.0 2.3 ZT66 有 淤泥质黏土 4.3 1.7 ZT23 有 淤泥质粉质黏土 14.0 2.0 ZT68 有 淤泥、淤泥质黏土 4.0 6.1 ZT24 有 淤泥质粉质黏土 14.0 1.5 ZT69 有 淤泥质粉质黏土 10.5 3.0 ZT26 有 淤泥质黏土 10.1 2.9 ZT70 有 淤泥质粉质黏土 16.0 2.3 ZT28 有 淤泥质黏土 7.9 3.6 ZT74 有 淤泥质粉质黏土、淤泥质黏土 14.0 3.5 ZT29 有 淤泥质黏土 13.9 2.5 ZT75 有 淤泥质粉质黏土 4.9 8.5 ZT30 有 淤泥质黏土 8.8 4.3 ZT76 有 淤泥质粉质黏土、淤泥质黏土 5.8 7.5 ZT31 有 淤泥质粉质黏土 6.7 4.8 ZT77 有 淤泥质粉质黏土 5.0 9.5 ZT32 有 淤泥、淤泥质粉质黏土 7.0 8.0 ZT78 有 淤泥质粉质黏土、淤泥质黏土 4.5 3.1 ZT33 有 淤泥质黏土 10.0 6.0 ZT79 有 淤泥质黏土 14.0 1.0 ZT34 有 淤泥质黏土 13.0 2.0 ZT80 有 淤泥质粉质黏土 11.7 3.3 ZT35 有 淤泥、淤泥质粉质黏土、淤泥质黏土 7.8 7.2 ZT81 有 淤泥质粉质黏土 5.6 4.4 ZT36 有 淤泥质粉质黏土 3.4 4.4 ZT82 有 淤泥质粉质黏土、淤泥质黏土 8.0 3.7 ZT37 有 淤泥质粉质黏土、淤泥质黏土 7.0 5.5 ZT83 有 淤泥质黏土 14.4 1.6 ZT38 有 淤泥质粉质黏土、淤泥质黏土 8.7 7.8 ZT85 有 淤泥质粉质黏土 12.0 1.7 ZT39 有 淤泥质黏土 9.0 1.0 ZT86 有 淤泥质黏土 11.6 2.4 ZT40 有 淤泥质粉质黏土、淤泥质黏土 10.5 6.0 ZT87 有 淤泥质粉质黏土 8.8 3.2 ZT41 有 淤泥质粉质黏土、淤泥质黏土 0.0 6.4 ZT89 有 淤泥质粉质黏土 9.8 2.7 ZT42 有 淤泥质粉质黏土 8.0 5.5 ZT90 有 淤泥质黏土 11.0 4.0 ZT43 有 淤泥质黏土 9.0 3.0 ZT91 有 淤泥质粉质黏土 6.6 2.4 ZT44 有 淤泥质粉质黏土、淤泥质黏土 6.1 11.7 ZT92 有 淤泥质粉质黏土 13.0 3.0 注:刁口乡行政上不隶属于河口区,本次未进行钻孔布设,故亦未开展软土的圈定。 2.3 钻孔剖面地层分布
据本次92个工程地质钻探孔剖面(图10)及岩芯统计分析,河口区地下50 m内第四系全新统冲积海积物岩性以粉土最为广泛,其次为粉质黏土、粉砂、黏土,局部有细砂,上部多为土黄—灰黄色粉土、粉质黏土;中部为灰黑色粉质黏土或淤泥质土,具腥味;下部多为浅灰色粉砂土层,其物理力学性质在水平和垂向上均有较大的变化,揭露6个主层,1层为素填土,2层为第一陆相层,3层为第一海相层,4层为第二陆相层,5层为第二海相层,6层为第三陆相层。1—4层为30 m以浅地层,5—6层是30~50 m地层。
2.4 物质成分结构、成因
河口区地下水位皆较浅,本次钻探场区50.0 m深度范围内地下水为第四系孔隙潜水-微承压水,地下水位埋深0.20~4.30 m,水位标高−1.30~5.44 m,水位变幅1.00~3.00 m。河口地区形成的软土主要为新近沉积的淤泥质黏土、淤泥质粉质黏土以及淤泥,其物质成分主要是粉质亚黏土,除部分石英、长石矿物外,还含有大量黏土矿物,并含有少量的水溶盐类矿物,有机质含量较多。从外观上看,常呈灰色、灰绿和灰黑等暗色,污染手指,伴有臭味,结构形式多为蜂窝状或海绵状,疏松多孔,被扰动后,结构已破坏,强度降低,呈现流塑状态。定向排列明显,层理较发育,具薄层构造,常含有粉砂夹层或泥炭透镜体。根据该区域最新的同位素年龄,属全新世新近沉积土,是由于黄河按照淤积→延伸→抬高→摆动→改道规律经历的历次河道摆动迁移淤积,同时不断向海淤进,在河流和海水双重作用下,形成陆海相交互的连续沉积体[6-7, 27]。
2.5 沉积环境
按形成和分布情况,我国软土类土基本上可以分为两大类:一类是沿海沉积的淤泥类土(瀉湖相、溺谷相、滨海相、三角洲相);一类是内陆和山区湖盆地及山前谷地沉积的淤泥类土。
黄河三角洲河口地区为沿海沉积的淤泥类土,具体可分为滨海相、三角洲相软土。
滨海相软土的分布受古地形、古海岸线的控制,主要分布在河口地区靠近沿海的地段,海相与陆相地层交替沉积,有软土形成的环境条件和物质来源,沉积物表层多为数米的褐黄色亚黏土,以下为含淤泥质黏性土或淤泥质黏性土,常夹带有粉砂薄层或透镜体,这种粉砂夹层是由黏土或粉砂交替形成,呈细微条带状构造,含有海生物,具有向海倾斜的斜层理[7, 24]。
三角洲相软土主要分布在相对靠南的内陆部分,其分布与地形地貌、水文地质等条件关系密切,分布地段在地貌上处于滨海平原区和冲积平原与滨海平原的交接地带及黄河三角洲平原区,软土层分布宽阔,厚度相对比较稳定,由于受滨海海流与波浪的影响,分选程度较差,多有交错斜层理或不规则的透镜体夹层,地势平缓,地下水、地表水排泄不畅,在这种特殊的滨海三角洲环境中,河口区沉积了水平层理发育良好的软弱黏性土[6-7, 24]。
2.6 软土不良问题及工程处理措施
黄河三角洲河口地区软土不良土体的主要工程地质问题表现在两个方面:一是该地区地处广泛的河口三角洲冲积平原区,在新近沉积的土层上部荷载压力作用下,土层自固结沉降量很大,且沉降不均匀。软土层在河口地区的大面积分布,是该区域地面沉降自固结地面沉降的主要贡献层,由于其渗透性低,孔隙水排出过程相当缓慢,因此,其土层的固结过程亦很缓慢,有的地基甚至几十年后仍可能有沉降发生[3, 6]。二是该地区软土层尤其是靠近沿海的滨海地段,软土层分布不均匀厚度稳定,多具向海倾斜的斜层理或呈透镜体状,易引起建筑物地基不均匀沉降,发生剪切破坏或倾斜挤出,这在港口、码头地段尤其要引起建设规划部门的重视[6]。
针对河口地区软土工程特性存在的不良工程地质问题,建议采取以下地基处理方法:
(1)桩基法。在深厚的软土地基上,当建筑物的荷载较大,或对地基变形和稳定性要求较高,而采用其他处理措施不能满足要求时,宜采用桩基方案。桩基具有承载力高、沉降量小和沉降较为均匀等优点,因而在软土地区得到广泛应用。
(2)排淤换填法。由于河口地区靠近沿海,在沿海地段,有大量的围垦、堤防、港口、码头等,在这些地方,可以利用抛石体的自重下沉到相对稳定的土层中,以抛石体护底形成建筑物地基支撑围堤、堆场等上部结构来加固地层,对于地基中有较厚软土层而又无法完全清除的情况,可挖去一定深度的软土层,代之以人工填筑的砂石层,以扩大基础底面积,提高地基的强度。
(3)堆载预压法。适用于处理深厚软土地基,采用预定荷载加压,使之固结,一般在地基中打入砂井或塑料排水带对软土进行堆载预压、加速排水固结,提高淤泥或淤泥质土的强度,但要注意加荷总量和速度都不易过大,以免地基失稳。
(4)电化学加固法。电渗排水和硅化加固相结合,在压力双液硅化法的基础上设置电报通入直流电,在电渗作用下,孔隙水由阳极流向阴极,化学溶液也随之渗流分布于土的孔隙中,经化学反应后生成硅胶,经过电渗作用还可以使硅胶部分脱水,加速加固过程,并增加其强度。
3. 结论
(1)黄河三角洲北部河口地区软土具有典型的软土特征:含水量高、孔隙比高、压缩性高、透水性低、低抗剪强度、低承载力等,其物理力学性质指标变异系数较小,数据稳定性较好,指标数据统计基本服从正态曲线分布规律,具有较好的代表性,分析研究结果可靠程度较高,软土各类物理力学性质指标间的相关性较为显著。
(2)黄河三角洲河口地区软土的空间分布,从垂向上来说主要为分布在30 m以浅的第一陆相层及第一海相层的淤泥质黏土、淤泥质粉质黏土、淤泥,沉积环境主要为三角洲相和滨海相沉积两大类。靠南区域的三角洲相软土,分布范围相对较小,埋藏较浅,厚度较小;滨海相软土,分布范围较广,埋藏较深,厚度较大,具向海倾斜的斜层理。从平面上来说,整个河口区软土分布面积较大,占据了近85%的范围,滨海近岸地段皆为软土存在区域,在西部新户镇、义和镇及南部区域有部分零星无软土分布区。
(3)软土大面积存在会导致长时间累积的地面沉降及地基不均匀沉降等工程地质问题,换土垫层法、桩基法、电化学加固法等方法是处理软土不良工程问题的良好手段,建议加大这方面的研究及推广应用。
-
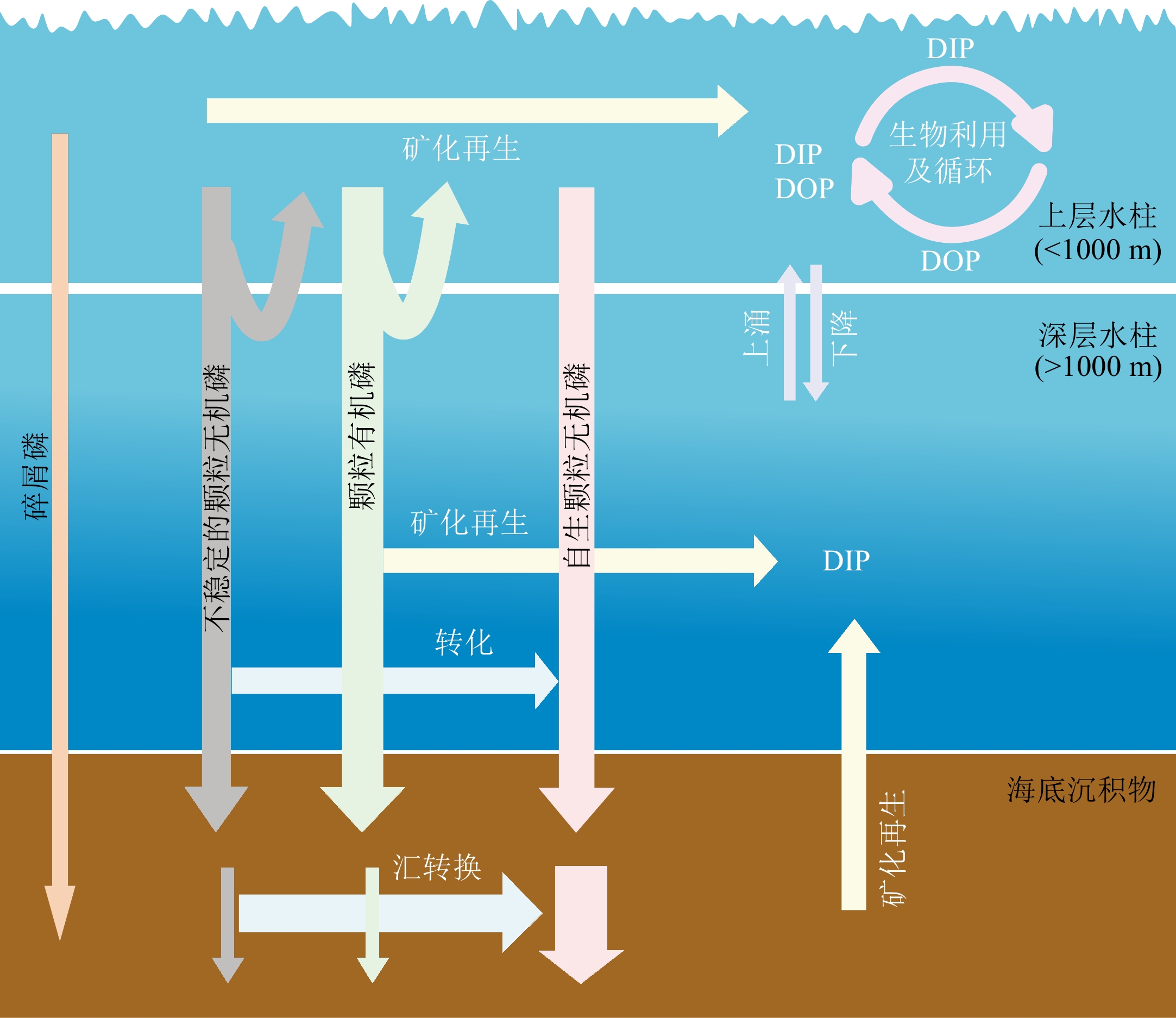
磷主要以碎屑磷、颗粒有机磷和颗粒无机磷等形式从表层水体流失。在沉降的过程中,颗粒态磷可以通过再矿化转化为溶解态,不稳定的颗粒无机磷可以转化为自生颗粒无机磷。沉降到海底沉积物中的有机磷部分可以矿化再生,不稳定的颗粒磷通过“汇转换”形成自生CFA(详细内容请见正文)。DIP:溶解无机磷,DOP:溶解有机磷。
Figure 1. Transformations between P pools in water column and sediments [9,14]
Phosphorus is lost from surface waters in mainly the forms of particulate organic P (POP), labile particulate inorganic P (labile PIP), and authigenic PIP. During the sedimentation, PIP and POP may undergo regeneration into DIP, and labile PIP can be transformed into authigenic PIP. In seafloor sediments, a fraction of POP can undergo regeneration, leading to the release of DIP into the seawater. Additionally, unstable forms of particulate phosphorus have the potential to be transformed into authigenic carbon fluorapatite (CFA) through the process known as “sink switching” (see the text for more details). DIP: dissolved inorganic phosphorus; DOP: dissolved organic phosphorus.
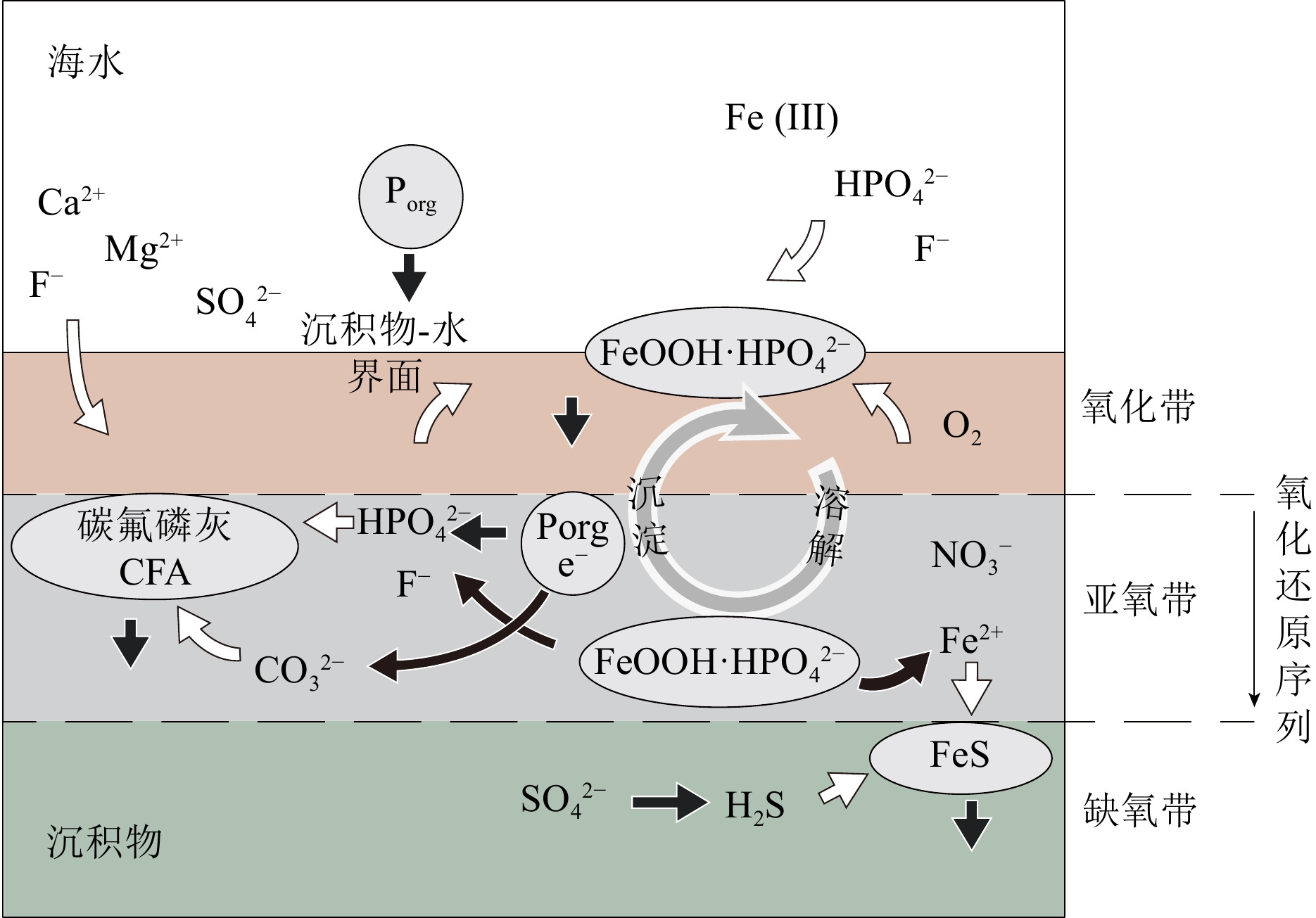
图 2 海洋沉积物中Porg 和PFe(FeOOH·HPO42−)向CFA转换[8]
在沉积物-水界面,FeOOH从海水中吸附HPO42−和F−。随着沉积物中有机质的分解,HPO42−、CO32− 被释放到孔隙水中,同时FeOOH还原溶解释放出Fe2+、HPO42− 和F−,导致孔隙水中这些离子的浓度增加,促使自生CFA的沉淀。FeOOH的还原产生的Fe2+向下在沉积物缺氧带与硫化物一起沉淀为FeS;向上在氧化带被重新氧化成FeOOH。有机质分解形成的HPO42−一部分会向沉积物-水界面扩散,被FeOOH重新吸收。
Figure 2. Schematic diagram of “sink switch” in marine sediments [8]
At the sediment-water interface, FeOOH can absorb HPO42− and F− from seawater. Upon the decomposition of organic matter, HPO42−and CO32− are released into the pore water. The reductive dissolution of FeOOH releases Fe2+, HPO42−, and F−, leading to an increase in their concentrations within pore waters and the subsequent precipitation of authigenic carbon fluorapatite (CFA). The reduction of FeOOH results in the production of Fe2+ ions, which can either precipitate as FeS in the anoxic zone or be re-oxidized back into FeOOH in the oxidized zone. HPO42− released from the decomposition of organic matter diffuses up toward the sediment-water interface, where it is reabsorbed by FeOOH.
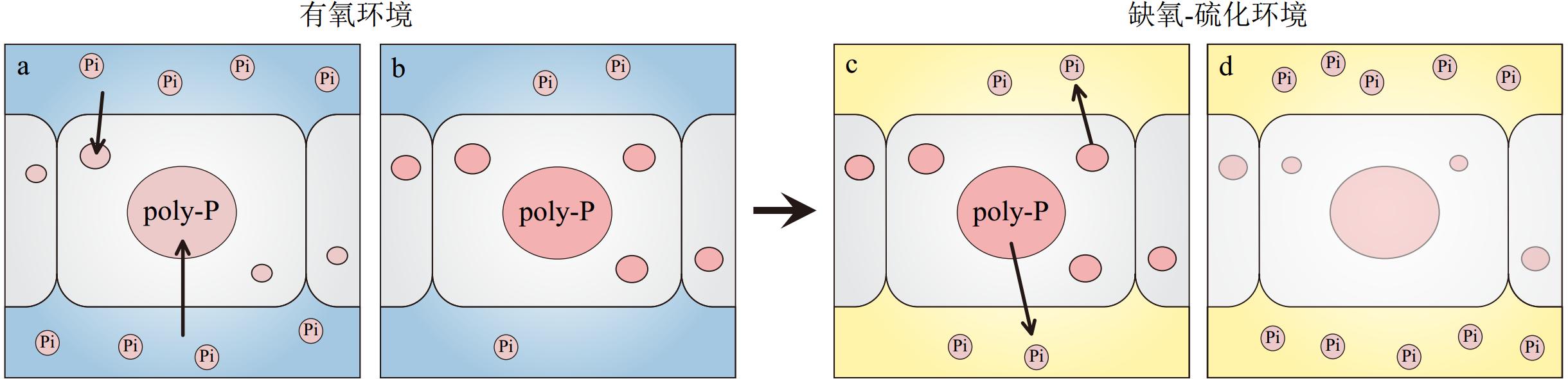
图 3 大型硫化细菌 Beggiatoa 对磷酸盐的吸收和释放[44]
a-b:在有氧、低硫化物浓度的环境下,Beggiatoa吸收磷酸盐并以poly-P的形式储存,环境中的磷酸盐浓度减少;c-d:在缺氧-硫化环境,Beggiatoa分解poly-P,并以磷酸盐的形式释放,环境中的磷酸盐浓度增加。Pi代表无机磷酸盐。
Figure 3. Proposed phosphate uptake by and release from Beggiatoa [44]
a-b: Under oxic conditions, phosphate is taken by Beggiatoa and accumulated as polyphosphate. c-d: When the conditions change to anoxia and exposure to sulfide increases, Beggiatoa decomposes polyphosphate and release phosphate. This leads to an increase in phosphate in the medium.
图 5 CFA交代方解石的图像[75]
左边的图像为扫描电子显微镜(SEM)图像,右边的图像为左图中对应点位的能量色散谱(EDS)。有孔虫的碳酸钙壳壁(深灰色)显示出被CFA交代的特征:CFA以隐晶质胶结物(CFA cement,白色)的形式存在于基质中,碳酸钙壳壁部分残余(深灰色);在有孔虫外壳上CFA沿平行于房室壁的孔洞依次排列,形成线脉状(CFA lining,浅灰色)。在有孔虫的空腔内CFA形成结晶体(CFA crystals,白色-浅灰色)。
Figure 5. Images of authigenic carbon fluorapatite (CFA) replacement of calcite in foraminifera tests [75]
The left panels represent the scanning electron microscopy (SEM) images and the right panels are Energy Dispersive Spectroscopy (EDS) point analyses. The calcium carbonate shell wall of foraminifera (dark gray) shows features of replacement by CFA: CFA is present in the matrix as cryptocrystalline cement (CFA cement, white), with partial remnants of the calcium carbonate shell wall (dark gray); on the foraminiferal shell CFA is sequentially arranged along the pores parallel to the atrial wall, forming a linear vein (CFA lining, light gray). CFA crystals (CFA crystals, white-light gray) in the cavity of foraminifera.
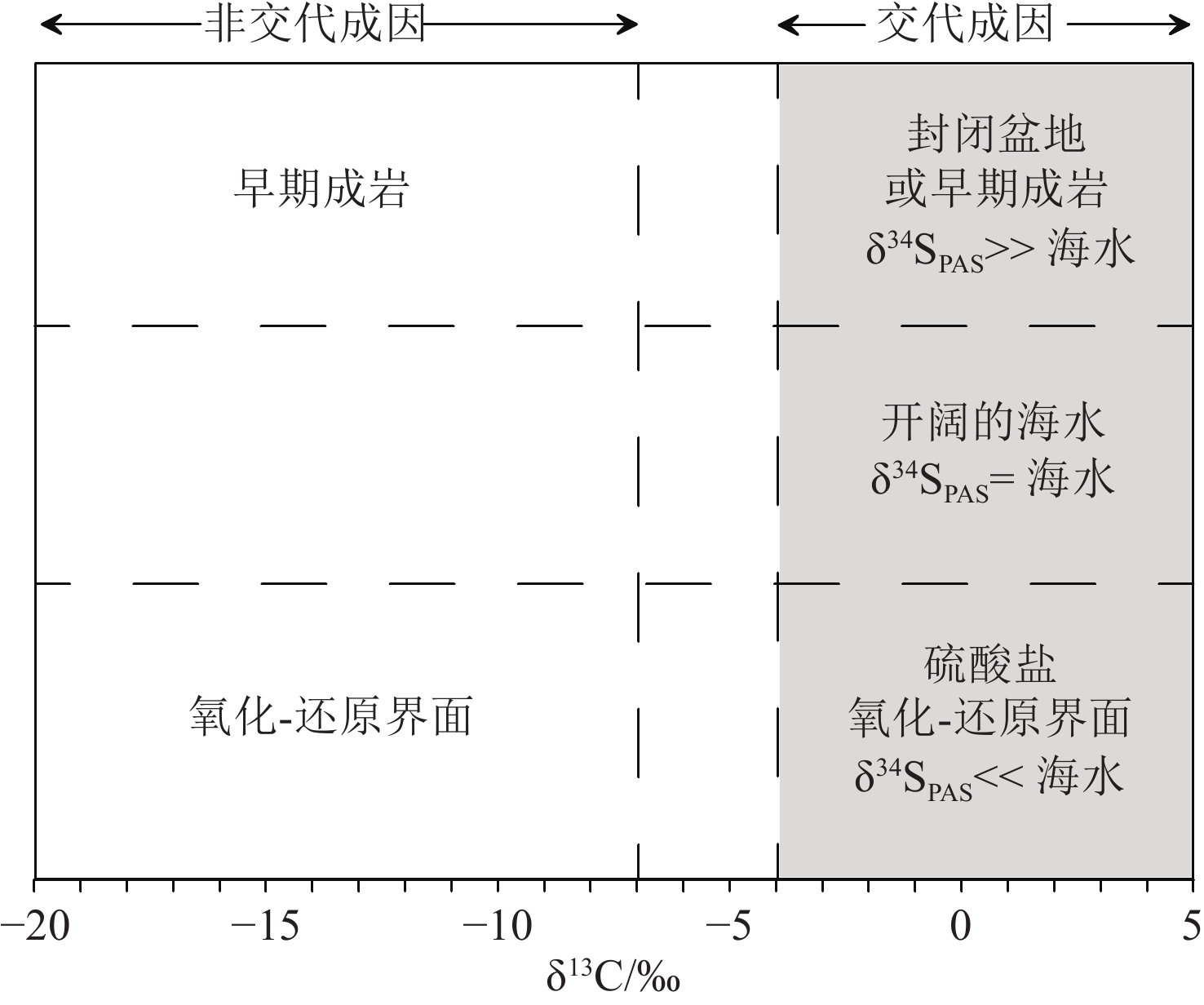
图 6 CFA的不同形成机制及形成环境[77]
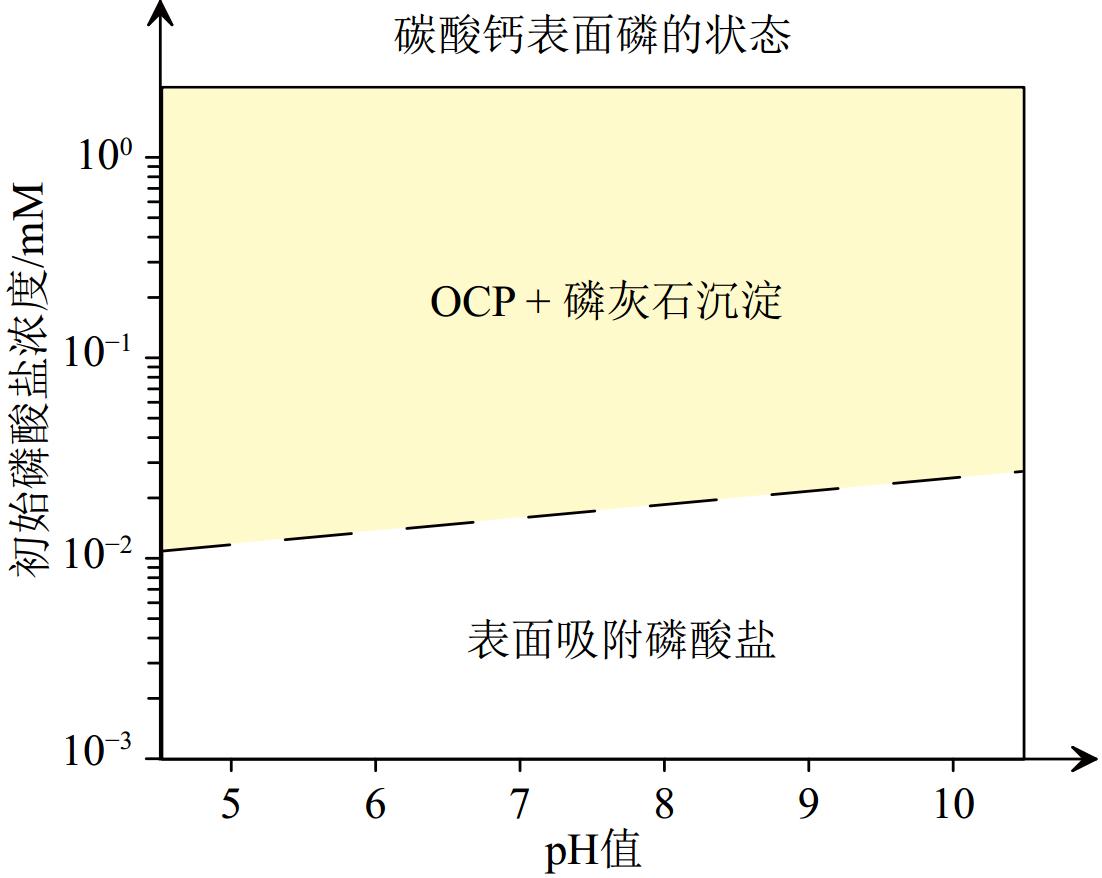
CFA的结构CO32– 的 δ13C 值为CFA的不同形成机制提供了鉴别依据,结构SO42–的 δ34SPAS值反映了CFA在沉积物中的形成环境。交代成因CFA既可以形成于硫酸盐还原条件下,又可以形成于开阔海水中或硫酸盐还原-氧化界面。在封闭盆地或早期成岩过程中,硫酸盐还原作用为主导,由于孔隙水与海水无法及时交换,导致剩余在孔隙水中的SO42–其δ34S偏重;而在硫酸盐氧化-还原界面,在大型硫化细菌的参与下形成的SO42–其δ34S偏轻(见2.4.1节)。
Figure 6. Different formation mechanisms and environments of authigenic carbon fluorapatite (CFA) [77]
The δ13C values of structural CO32- of CFA can be used to distinguish the precipitation mechanism of CFA. The δ34SPAS value of structural SO42- reflects the environment of CFA formation in the sediment. Alternative CFA can be formed under sulfate reduction conditions, as well as in open seawater or at sulfate reduction-oxidation interfaces (see 2.4.1).
-
[1] Föllmi K B. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits[J]. Earth-Science Reviews, 1996, 40(1-2):55-124. doi: 10.1016/0012-8252(95)00049-6
[2] Ashley K, Cordell D, Mavinic D. A brief history of phosphorus: From the philosopher's stone to nutrient recovery and reuse[J]. Chemosphere, 2011, 84(6):737-746. doi: 10.1016/j.chemosphere.2011.03.001
[3] Tyrrell T. The relative influences of nitrogen and phosphorus on oceanic primary production[J]. Nature, 1999, 400(6744):525-531. doi: 10.1038/22941
[4] Bjerrum C J, Canfield D E. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides[J]. Nature, 2002, 417(6885):159-162. doi: 10.1038/417159a
[5] 周强, 姜允斌, 郝记华, 等. 磷的生物地球化学循环研究进展[J]. 高校地质学报, 2021, 27(2):183-199 doi: 10.16108/j.issn1006-7493.2020002 ZHOU Qiang, JIANG Yunbin, HAO Jihua, et al. Advances in the study of biogeochemical cycles of phosphorus[J]. Geological Journal of China Universities, 2021, 27(2):183-199.] doi: 10.16108/j.issn1006-7493.2020002
[6] McElroy M B. Marine biological controls on atmospheric CO2 and climate[J]. Nature, 1983, 302(5906):328-329. doi: 10.1038/302328a0
[7] Algeo T J, Ingall E. Sedimentary Corg: P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2007, 256(3-4):130-155. doi: 10.1016/j.palaeo.2007.02.029
[8] Ruttenberg K C. The global phosphorus cycle[M]//Treatise on Geochemistry. 2nd ed. Amsterdam: Elsevier, 2014, 10:499-558.
[9] Defforey D, Paytan A. Phosphorus cycling in marine sediments: Advances and challenges[J]. Chemical Geology, 2018, 477:1-11. doi: 10.1016/j.chemgeo.2017.12.002
[10] Froelich P N, Bender M L, Luedtke N A, et al. The marine phosphorus cycle[J]. American Journal of Science, 1982, 282(4):474-511. doi: 10.2475/ajs.282.4.474
[11] Arning E T, Lückge A, Breuer C, et al. Genesis of phosphorite crusts off Peru[J]. Marine Geology, 2009, 262(1-4):68-81. doi: 10.1016/j.margeo.2009.03.006
[12] Delaney M L. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle[J]. Global Biogeochemical Cycles, 1998, 12(4):563-572. doi: 10.1029/98GB02263
[13] Tribble J S, Arvidson R S, Lane M III, et al. Crystal chemistry, and thermodynamic and kinetic properties of calcite, dolomite, apatite, and biogenic silica: applications to petrologic problems[J]. Sedimentary Geology, 1995, 95(1-2):11-37. doi: 10.1016/0037-0738(94)00094-B
[14] Paytan A, McLaughlin K. The oceanic phosphorus cycle[J]. ChemInform, 2007, 38(20):563-576.
[15] Filippelli G M, Delaney M L. Phosphorus geochemistry of equatorial Pacific sediments[J]. Geochimica et Cosmochimica Acta, 1996, 60(9):1479-1495. doi: 10.1016/0016-7037(96)00042-7
[16] 刘世荣, 胡瑞忠, 周国富, 等. 织金新华磷矿碎屑磷灰石的矿物成分研究[J]. 矿物学报, 2008, 28(3):244-250 doi: 10.16461/j.cnki.1000-4734.2008.03.004 LIU Shirong, HU Ruizhong, ZHOU Guofu, et al. Study on the mineral composition of the clastic phosphate in Zhijin phosphate deposits, China[J]. Acta Mineralogica Sinica, 2008, 28(3):244-250.] doi: 10.16461/j.cnki.1000-4734.2008.03.004
[17] Hughes J M, Rakovan J F. Structurally robust, chemically diverse: apatite and apatite supergroup minerals[J]. Elements, 2015, 11(3):165-170. doi: 10.2113/gselements.11.3.165
[18] Jarvis I. Phosphorite geochemistry: state-of-the-art and environmental concerns[J]. Eclogae Geologicae Helvetiae, 1994, 87(3):643-700.
[19] Pan Y, Fleet M E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors[J]. Reviews in Mineralogy & Geochemistry, 2002, 48(1):13-49.
[20] Nathan Y, Sass E. Stability relations of apatites and calcium carbonates[J]. Chemical Geology, 1981, 34(1-2):103-111. doi: 10.1016/0009-2541(81)90075-9
[21] Piper D Z, Perkins R B. Geochemistry of a marine phosphate deposit: A signpost to phosphogenesis[M]//Treatise on Geochemistry. 2nd ed. Amsterdam Elsevier, 2014, 13: 293-312.
[22] Ruttenberg K C. Development of a sequential extraction method for different forms of phosphorus in marine sediments[J]. Limnology and Oceanography, 1992, 37(7):1460-1482. doi: 10.4319/lo.1992.37.7.1460
[23] Ruttenberg K C, Berner R A. Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments[J]. Geochimica et Cosmochimica Acta, 1993, 57(5):991-1007. doi: 10.1016/0016-7037(93)90035-U
[24] Benitez-Nelson R C. The biogeochemical cycling of phosphorus in marine systems[J]. Earth-Science Reviews, 2000, 51(1-4):109-135. doi: 10.1016/S0012-8252(00)00018-0
[25] Filippelli G M. Phosphate rock formation and marine phosphorus geochemistry: The deep time perspective[J]. Chemosphere, 2011, 84(6):759-766. doi: 10.1016/j.chemosphere.2011.02.019
[26] Faul K L, Paytan A, Delaney M L. Phosphorus distribution in sinking oceanic particulate matter[J]. Marine Chemistry, 2005, 97(3-4):307-333. doi: 10.1016/j.marchem.2005.04.002
[27] Ruttenberg K C. Reassessment of the oceanic residence time of phosphorus[J]. Chemical Geology, 1993, 107(3-4):405-409. doi: 10.1016/0009-2541(93)90220-D
[28] Howarth R W, Jensen H, Marino R, et al. Transport to and processing of P in near-shore and oceanic waters[M]//Phosphorus in the Global Environment. Chichester: John Wiley & Sons, 1995, 54:323-345.
[29] Slomp C P, Epping E H G, Helder W, et al. A key role for iron-bound phosphorus in authigenic apatite formation in North Atlantic continental platform sediments[J]. Journal of Marine Research, 1996, 54(6):1179-1205. doi: 10.1357/0022240963213745
[30] Froelich P N, Klinkhammer G P, Bender M L, et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis[J]. Geochimica et Cosmochimica Acta, 1979, 43(7):1075-1090. doi: 10.1016/0016-7037(79)90095-4
[31] Glud R N. Oxygen dynamics of marine sediments[J]. Marine Biology Research, 2008, 4(4):243-289. doi: 10.1080/17451000801888726
[32] 郑旻, 罗敏, 潘彬彬, 等. 海洋沉积物溶解氧消耗研究进展[J]. 地球科学进展, 2023, 38(3):236-255 ZHENG Min, LUO Min, PAN Binbin, et al. Research progress of oxygen consumption in marine sediments[J]. Advances in Earth Science, 2023, 38(3):236-255.]
[33] Suess E. Phosphate regeneration from sediments of the Peru continental margin by dissolution of fish debris[J]. Geochimica et Cosmochimica Acta, 1981, 45(4):577-588. doi: 10.1016/0016-7037(81)90191-5
[34] Schenau S J, De Lange G J. Phosphorus regeneration vs. burial in sediments of the Arabian Sea[J]. Marine Chemistry, 2001, 75(3):201-217. doi: 10.1016/S0304-4203(01)00037-8
[35] Omelon S, Ariganello M, Bonucci E, et al. A review of phosphate mineral nucleation in biology and geobiology[J]. Calcified Tissue International, 2013, 93(4):382-396. doi: 10.1007/s00223-013-9784-9
[36] Atlas E, Culberson C, Pytkowicz R M. Phosphate association with Na+, Ca2+ and Mg2+ in seawater[J]. Marine Chemistry, 1976, 4(3):243-254. doi: 10.1016/0304-4203(76)90011-6
[37] Slomp C P, Van Cappellen P. The global marine phosphorus cycle: sensitivity to oceanic circulation[J]. Biogeosciences, 2007, 4(2):155-171. doi: 10.5194/bg-4-155-2007
[38] Froelich P N, Arthur M A, Burnett W C, et al. Early diagenesis of organic matter in Peru continental margin sediments: Phosphorite precipitation[J]. Marine Geology, 1988, 80(3-4):309-343. doi: 10.1016/0025-3227(88)90095-3
[39] Schulz H N, Schulz H D. Large sulfur bacteria and the formation of phosphorite[J]. Science, 2005, 307(5708):416-418. doi: 10.1126/science.1103096
[40] Arning E T. Phosphogenesis in coastal upwelling systems—bacterially-induced phosphorite formation[D]. Bremen: Technische Universität Bergakademie Freiberg, 2008.
[41] Berndmeyer C, Birgel D, Brunner B, et al. The influence of bacterial activity on phosphorite formation in the Miocene Monterey Formation, California[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2012, 317-318:171-181. doi: 10.1016/j.palaeo.2012.01.004
[42] Cosmidis J, Benzerara K, Menguy N, et al. Microscopy evidence of bacterial microfossils in phosphorite crusts of the Peruvian shelf: Implications for phosphogenesis mechanisms[J]. Chemical Geology, 2013, 359:10-22. doi: 10.1016/j.chemgeo.2013.09.009
[43] Diaz J, Ingall E, Benitez-Nelson C, et al. Marine polyphosphate: A key player in geologic phosphorus sequestration[J]. Science, 2008, 320(5876):652-655. doi: 10.1126/science.1151751
[44] Brock J, Schulz-Vogt H N. Sulfide induces phosphate release from polyphosphate in cultures of a marine Beggiatoa strain[J]. The ISME Journal, 2011, 5(3):497-506. doi: 10.1038/ismej.2010.135
[45] Mänd K, Kirsimäe K, Lepland A, et al. Authigenesis of biomorphic apatite particles from Benguela upwelling zone sediments off Namibia: The role of organic matter in sedimentary apatite nucleation and growth[J]. Geobiology, 2018, 16(6):640-658. doi: 10.1111/gbi.12309
[46] Williams L A, Reimers C. Role of bacterial mats in oxygen-deficient marine basins and coastal upwelling regimes: Preliminary report[J]. Geology, 1983, 11(5):267-269. doi: 10.1130/0091-7613(1983)11<267:ROBMIO>2.0.CO;2
[47] Arning E T, Birgel D, Brunner B, et al. Bacterial formation of phosphatic laminites off Peru[J]. Geobiology, 2009, 7(3):295-307. doi: 10.1111/j.1472-4669.2009.00197.x
[48] Lumiste K, Mänd K, Bailey J, et al. Constraining the conditions of phosphogenesis: Stable isotope and trace element systematics of Recent Namibian phosphatic sediments[J]. Geochimica et Cosmochimica Acta, 2021, 302:141-159. doi: 10.1016/j.gca.2021.03.022
[49] McArthur J M, Benmore R A, Coleman M L, et al. Stable isotopic characterisation of francolite formation[J]. Earth and Planetary Science Letters, 1986, 77(1):20-34. doi: 10.1016/0012-821X(86)90129-9
[50] Paytan A, Kastner M, Campbell D, et al. Sulfur isotopic composition of Cenozoic seawater sulfate[J]. Science, 1998, 282(5393):1459-1462. doi: 10.1126/science.282.5393.1459
[51] 马芮, 苏莉, 宋宇昊, 等. 多聚磷酸盐: 菌体内多功能调控子和环境压力守护者[J]. 微生物学通报, 2017, 44(7):1736-1746 MA Rui, SU Li, SONG Yuhao, et al. Inorganic polyphosphate: the multifunctional regulator and the guardian of environmental stresses in bacteria[J]. Microbiology China, 2017, 44(7):1736-1746.]
[52] Goldhammer T, Brüchert V, Ferdelman T G, et al. Microbial sequestration of phosphorus in anoxic upwelling sediments[J]. Nature Geoscience, 2010, 3(8):557-561. doi: 10.1038/ngeo913
[53] Lomnitz U, Sommer S, Dale A W, et al. Benthic phosphorus cycling in the Peruvian oxygen minimum zone[J]. Biogeosciences, 2016, 13(5):1367-1386. doi: 10.5194/bg-13-1367-2016
[54] Glock N, Romero D, Roy A S, et al. A hidden sedimentary phosphate pool inside benthic foraminifera from the Peruvian upwelling region might nucleate phosphogenesis[J]. Geochimica et Cosmochimica Acta, 2020, 289:14-32. doi: 10.1016/j.gca.2020.08.002
[55] LeKieffre C, Bernhard J M, Mabilleau G, et al. An overview of cellular ultrastructure in benthic foraminifera: New observations of rotalid species in the context of existing literature[J]. Marine Micropaleontology, 2018, 138:12-32. doi: 10.1016/j.marmicro.2017.10.005
[56] Crawford R M. The protoplasmic ultrastructure of the vegetative cell of Melosira varians C. A. Agardh[J]. Journal of Phycology, 1973, 9(1):50-61. doi: 10.1111/j.0022-3646.1973.00050.x
[57] Ruttenberg K C, Dyhrman S T. Temporal and spatial variability of dissolved organic and inorganic phosphorus, and metrics of phosphorus bioavailability in an upwelling-dominated coastal system[J]. Journal of Geophysical Research:Oceans, 2005, 110(C10):C10S13.
[58] 张明亮, 郭伟, 沈俊, 等. 古海洋氧化还原地球化学指标研究新进展[J]. 地质科技情报, 2017, 36(4):95-106 doi: 10.19509/j.cnki.dzkq.2017.0412 ZHANG Mingliang, GUO Wei, SHEN Jun, et al. New progress on geochemical indicators of ancient oceanic redox condition[J]. Geological Science and Technology Information, 2017, 36(4):95-106.] doi: 10.19509/j.cnki.dzkq.2017.0412
[59] Gächter R, Meyer J S, Mares A. Contribution of bacteria to release and fixation of phosphorus in lake sediments[J]. Limnology and Oceanography, 1988, 33(6):1542-1558.
[60] Ingall E, Jahnke R. Influence of water-column anoxia on the elemental fractionation of carbon and phosphorus during sediment diagenesis[J]. Marine Geology, 1997, 139(1-4):219-229. doi: 10.1016/S0025-3227(96)00112-0
[61] Krajewski K P, van Cappellen P, Trichet J, et al. Biological processes and apatite formation in sedimentary environments[J]. Eclogae Geologicae Helvetiae, 1994, 87(3):701-745.
[62] Borkiewicz O, Rakovan J, Cahill C L. Time-resolved in situ studies of apatite formation in aqueous solutions[J]. American Mineralogist, 2010, 95(8-9):1224-1236. doi: 10.2138/am.2010.3168
[63] Wang L J, Nancollas G H. Calcium orthophosphates: crystallization and dissolution[J]. Chemical Reviews, 2008, 108(11):4628-4669. doi: 10.1021/cr0782574
[64] Cappellen P V, Berner R A. Fluorapatite crystal growth from modified seawater solutions[J]. Geochimica et Cosmochimica Acta, 1991, 55(5):1219-1234. doi: 10.1016/0016-7037(91)90302-L
[65] Gunnars A, Blomqvist S, Martinsson C. Inorganic formation of apatite in brackish seawater from the Baltic Sea: an experimental approach[J]. Marine Chemistry, 2004, 91(1-4):15-26. doi: 10.1016/j.marchem.2004.01.008
[66] Rokidi S, Combes C, Koutsoukos P G. The calcium phosphate-calcium carbonate system: Growth of Octa calcium phosphate on calcium carbonates[J]. Crystal Growth & Design, 2011, 11(5):1683-1688.
[67] Wang L J, Ruiz-Agudo E, Putnis C V, et al. Kinetics of calcium phosphate nucleation and growth on calcite: implications for predicting the fate of dissolved phosphate species in alkaline soils[J]. Environmental Science & Technology, 2012, 46(2):834-842.
[68] Ren C, Li Y F, Zhou Q, et al. Phosphate uptake by calcite: Constraints of concentration and pH on the formation of calcium phosphate precipitates[J]. Chemical Geology, 2021, 579:120365. doi: 10.1016/j.chemgeo.2021.120365
[69] Sundby B, Gobeil C, Silverberg N, et al. The phosphorus cycle in coastal marine sediments[J]. Limnology & Oceanography, 1992, 37(6):1129-1145.
[70] Xu N, Yin H W, Chen Z G, et al. Mechanisms of phosphate retention by calcite: effects of magnesium and pH[J]. Journal of Soils and Sediments, 2014, 14(3):495-503. doi: 10.1007/s11368-013-0807-y
[71] Xu N, Chen M, Zhou K R, et al. Retention of phosphorus on calcite and dolomite: speciation and modeling[J]. RSC Advances, 2014, 4(66):35205-35214. doi: 10.1039/C4RA05461J
[72] Lamboy M. Phosphatization of calcium carbonate in phosphorites: microstructure and importance[J]. Sedimentology, 1993, 40(1):53-62. doi: 10.1111/j.1365-3091.1993.tb01090.x
[73] 潘家华, 刘淑琴, 罗照华, 等. 太平洋海山磷酸盐的产状、特征及成因意义[J]. 矿床地质, 2007, 26(2):195-203 doi: 10.16111/j.0258-7106.2007.02.006 PAN Jiahua, LIU Shuqin, LUO Zhaohua, et al. Modes of occurrence and characteristics of phosphorates on Pacific Guyots and their genetic significance[J]. Mineral Deposits, 2007, 26(2):195-203.] doi: 10.16111/j.0258-7106.2007.02.006
[74] Benninger L M, Hein J R. Diagenetic evolution of seamount phosphate[M]//Glenn C R, Prévôt L, Lucas J. Marine Authigenesis: from Global to Microbial. Tulsa, Oklahoma: SEPM, 2000:245-256.
[75] Benites M, Hein J R, Mizell K, et al. Miocene phosphatization of rocks from the summit of Rio Grande Rise, southwest Atlantic Ocean[J]. Paleoceanography and Paleoclimatology, 2021, 36(9):e2020PA004197. doi: 10.1029/2020PA004197
[76] McArthur J M, Coleman M L, Bremner J M. Carbon and oxygen isotopic composition of structural carbonate in sedimentary francolite[J]. Journal of the Geological Society, 1980, 137(6):669-673. doi: 10.1144/gsjgs.137.6.0669
[77] Benmore R A, Coleman M L, McArthur J M. Origin of sedimentary francolite from its sulphur and carbon isotope composition[J]. Nature, 1983, 302(5908):516-518. doi: 10.1038/302516a0
[78] 潘家华, 刘淑琴, 杨忆, 等. 太平洋水下海山磷酸盐的成因及形成环境[J]. 地球学报, 2004, 25(4):453-458 PAN Jiahua, LIU Shuqin, YANG Yi, et al. The origin and formation environment of phosphates on submarine guyots of the Pacific Ocean[J]. Acta Geoscientica Sinica, 2004, 25(4):453-458.]
[79] Birch G F. A model of penecontemporaneous phosphatization by diagenetic and authigenic mechanisms from the western margin of southern Africa[M]//Bentor Y. Marine Phosphorites—Geochemistry, Occurrence, Genesis. Jerusalem, Israel: SEPM, 1980: 70-100.




 下载:
下载: