Advancements in studying the biogeochemistry of methane in marine depositional systems through trace element geochemistry
-
摘要:
由地质过程与微生物作用共同塑造的地球环境,当前正受到全球变暖的威胁,其中甲烷作为一种极为重要的温室气体,对全球变暖的贡献率已经达到了20%。海洋沉积物是地球最大的甲烷储库,在海洋富甲烷环境,微生物参与的产甲烷、甲烷厌氧氧化和甲烷有氧氧化过程广泛存在,是研究错综复杂的甲烷生物地球化学循环过程的理想实验室。本文从地质微生物学角度解析了含微量元素的酶或辅酶介导的甲烷循环过程,梳理了微生物潜在的微量元素需求,并重点综述了近年来主要涉及海洋甲烷循环过程研究的微量元素和同位素地球化学证据。由于参与甲烷循环过程的微生物纯培养相对困难,而地球化学研究又难以实现对生物地球化学过程的精细刻画,微生物学与地球化学的学科交叉研究优势明显、前景广阔。阐明海洋富甲烷环境微生物活动与微量元素的耦合关系,对于探索当前全球变暖背景下海洋甲烷循环过程和全球甲烷排放的调控至关重要,也有望为解析地质历史时期的甲烷排放事件及其全球生态环境效应提供独特的视角。
Abstract:The habitable planet, shaped by geological processes and microbial activity, is currently threatened by global warming. Methane, as an important greenhouse gas, is responsible for 20% of global warming. The largest amount of methane on the Earth is found in marine sediment. In these methane-rich marine environments, microbial process such as methanogenesis, anaerobic methane oxidation, and aerobic methane oxidation play a crucial role. In this review, the methane cycle mediated by enzymes or coenzymes containing trace elements was analyzed from the perspective of geological microbiology, the potential trace element demand of microorganisms was examined, and the geochemical evidence of trace elements and isotopes that primarily related to the study of the marine methane cycle in recent years were emphasized. At present, the pure culture of microorganisms involved in the methane cycle presents challenges, and to accurately describe biogeochemical processes in geochemical research is difficult. Therefore, interdisciplinary research that combines microbiology and geochemistry offers clear advantages and promising prospects. Understanding the interplay between microbial activities and trace elements in marine methane-rich environments is crucial for investigating the marine methane cycle and regulating global methane emissions in the context of current global warming. Additionally, this knowledge is anticipated to offer a distinctive vantage point for analyzing historical methane emission events and their global ecological/environmental impacts.
-
Keywords:
- marine depositional systems /
- methane cycle /
- microbial activity /
- trace elements
-
-
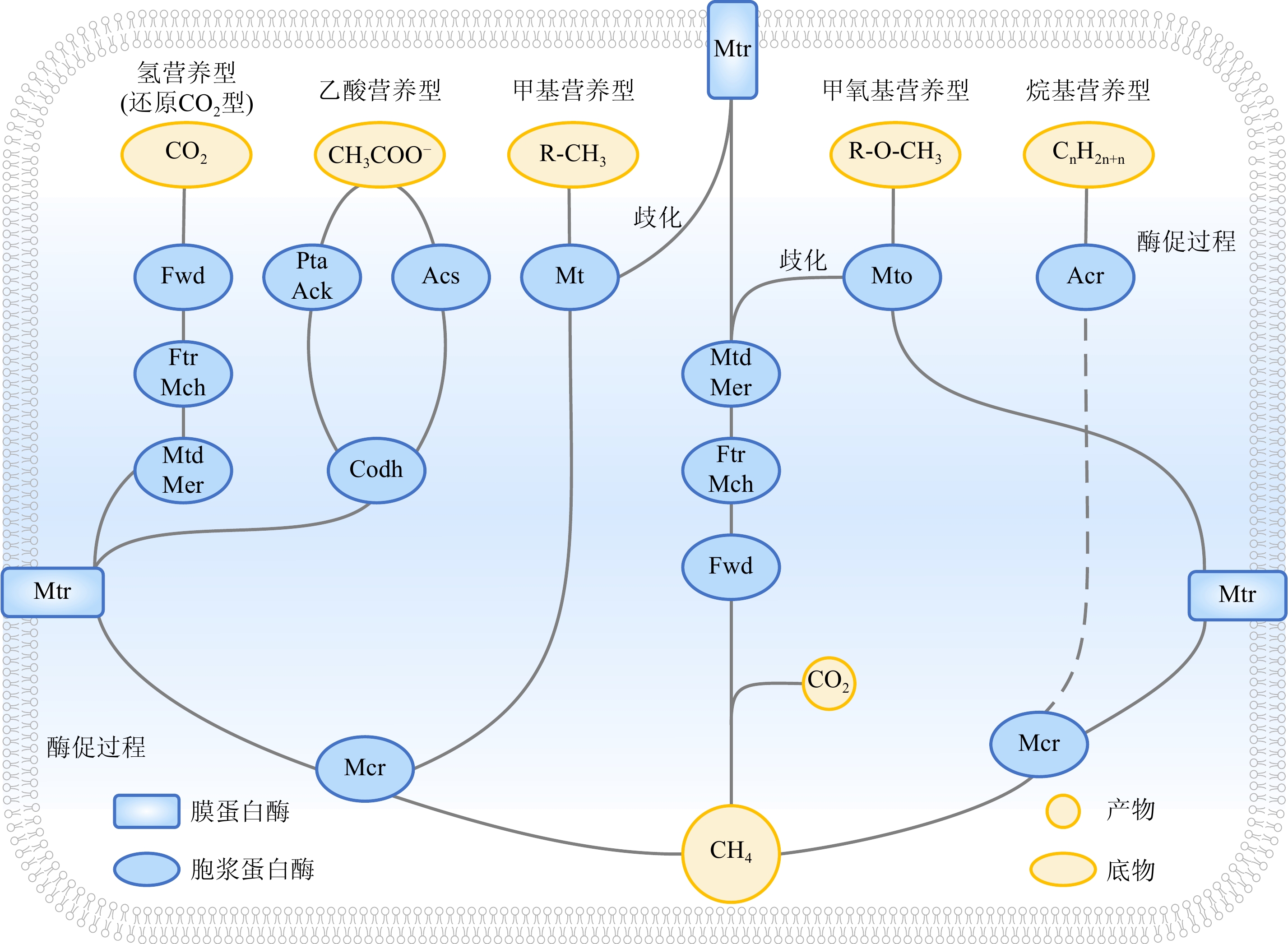
图 1 酶或辅酶介导的产甲烷途径 [24]
浅蓝色椭圆和长方形框内为促进产甲烷作用的各种酶,目前并不确定Ftr/Mch、Mtd/Mer和Pta/Ack是否会形成复合体,其他已知酶分别为:Fwd:甲酰甲烷呋喃脱氢酶;Mtr:四氢甲蝶呤S-甲基转移酶复合物;Mcr:甲基辅酶M还原酶; Acs:乙酰辅酶A合成酶; Codh:一氧化碳脱氢酶;Mt:甲基转移酶;Mto:辅酶M甲基转移酶系统;Acr:烷基辅酶M还原酶复合物。
Figure 1. Different pathways of methanogenesis mediated by enzyme or coenzyme [24]
The blue ovals and boxes contain various enzymes that promote methanogenesis. It is uncertain whether Ftr/Mch, Mtd/Mer, and Pta/Ack will form a complex at present, and other known enzymes are: Fwd: formyl-methanofuran dehydrogenase complex; Mtr: tetrahydromethanopterin S-methyl-transferase complex; Mcr: methyl-coenzyme M reductase complex; Acs: acetyl-CoA synthase; Codh: carbon monoxide dehydrogenase; Mt: methyltransferase; Mto: methoxyltransferase; Acr: alkyl-coenzyme M reductase complex.
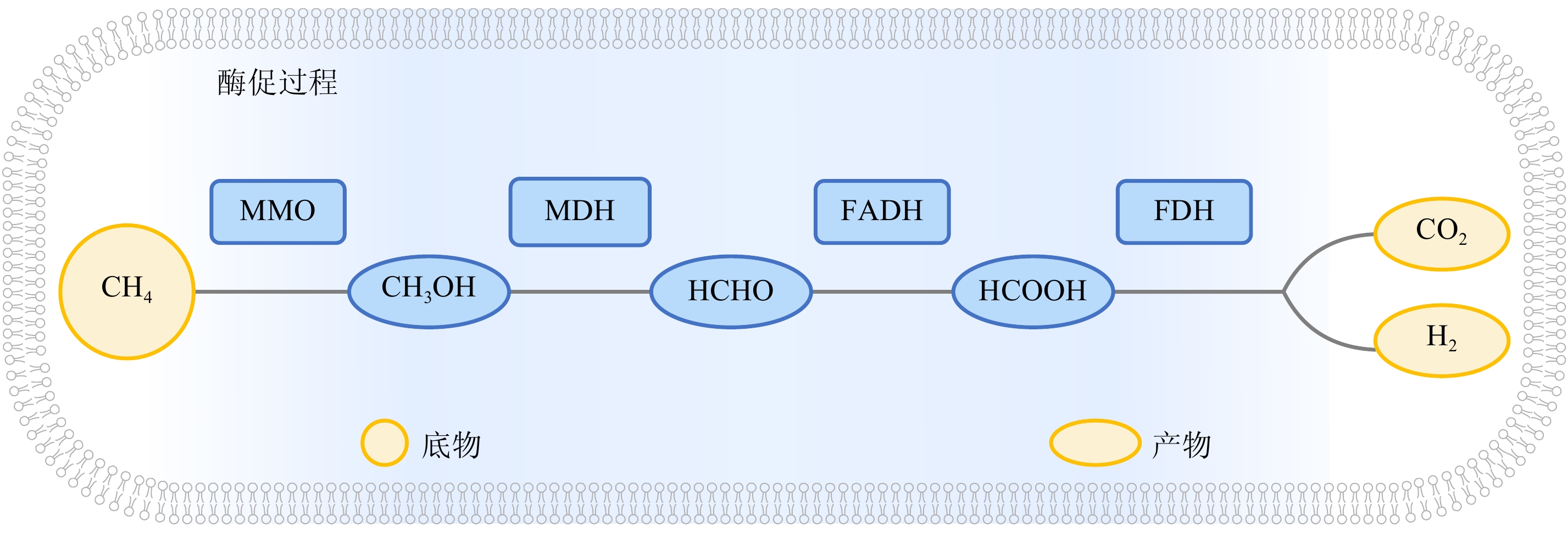
图 3 目前已知的甲烷有氧氧化过程
据文献[70]修改。浅蓝色长方形框内为促进反应的各种酶和中间产物。MMO:甲烷单加氧酶; MDH:甲醇脱氢酶; FADH:甲醛脱氢酶; FDH:甲酸脱氢酶。
Figure 3. Currently known process of aerobic oxidation of methane
Modified from reference [70]. Various enzymes and intermediates that promote this process are shown in the blue rectangular. MMO: methane monooxygenase; MDH: methanol dehydrogenase; FADH: formaldehyde dehydrogenase; FDH: formate dehydrogenase.
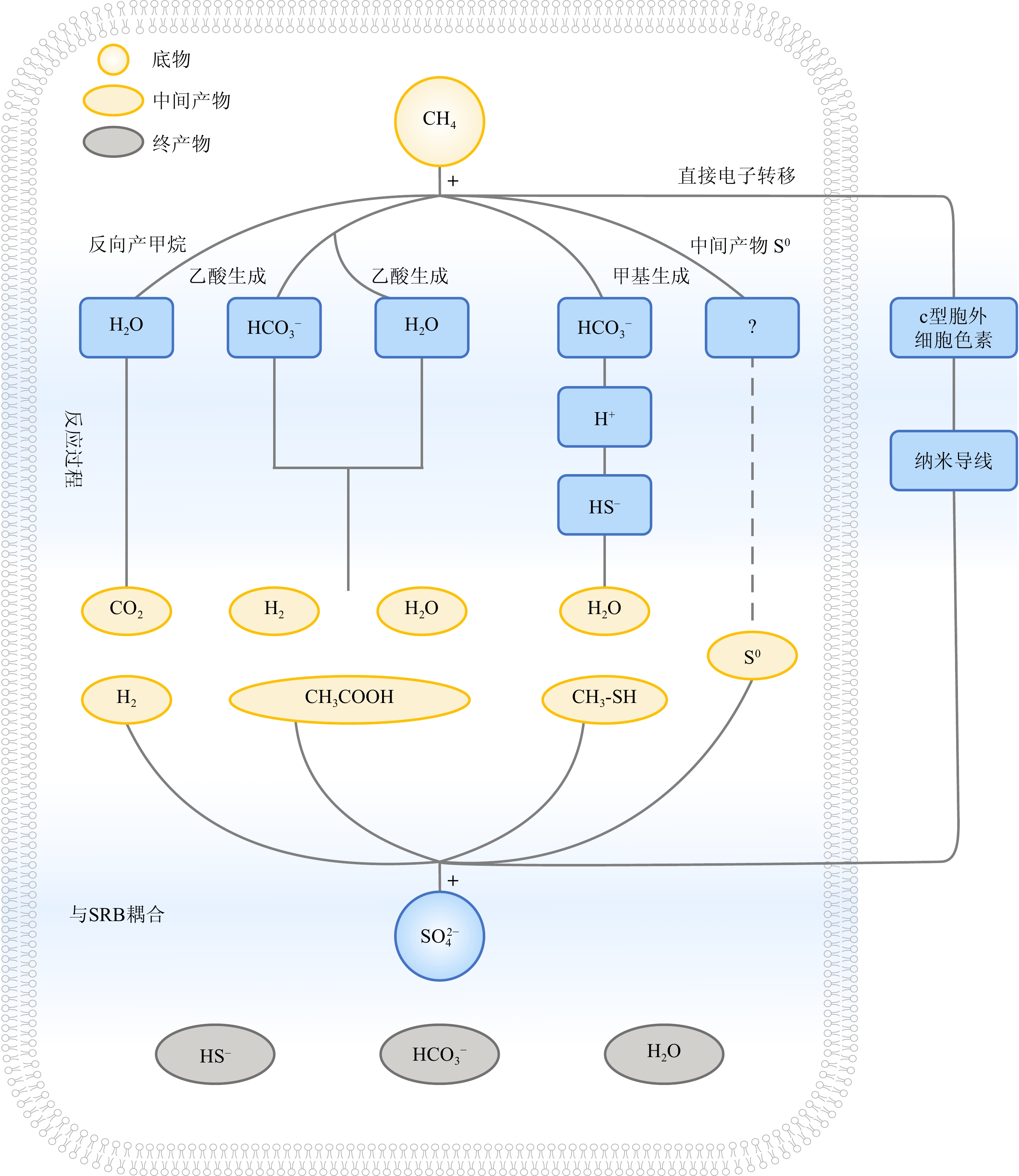
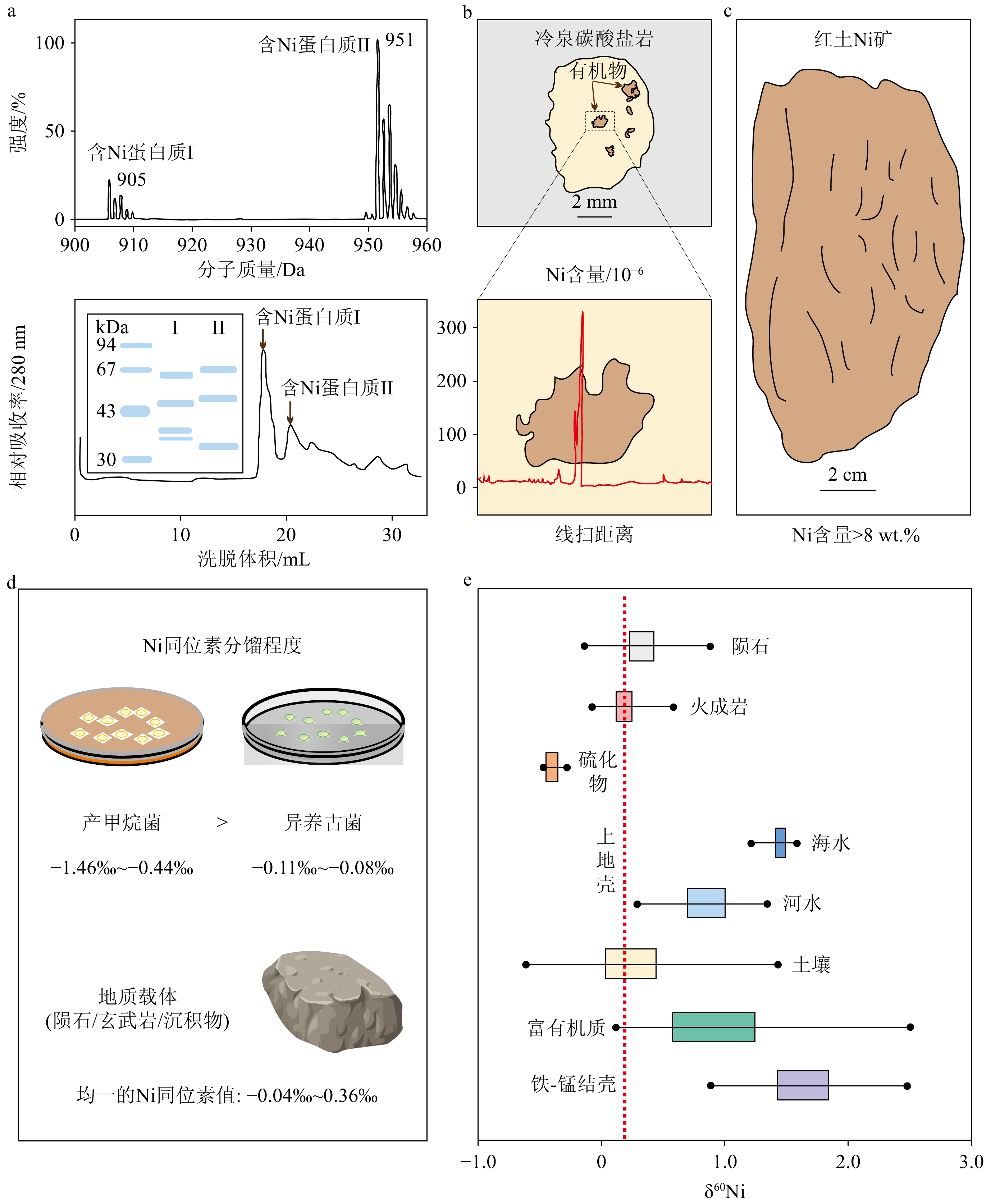
图 4 产甲烷和甲烷厌氧氧化作用的野外证据
a: 黑海冷泉细菌席两种含Ni蛋白质[19];b:法国南部晚阿普弟阶Marnes Bleues组地层出露的冷泉碳酸盐岩,富有机质区Ni含量剧增[86];c:甲烷循环过程的微生物成矿作用被认为一定程度上造就了哥伦比亚高Ni品味红土矿床,其中Ni含量最高达8 wt.%[85];d:产甲烷菌造成了大范围的Ni同位素分馏[88];e:Ni同位素指示产甲烷作用增强一定程度上驱动了6.35亿年前雪球地球的终止[92]。
Figure 4. Field evidence of methanogenesis and anaerobic oxidation of methane
a: two types of Ni-containing proteins were identified in the bacterial mats within the Black Sea cold seep area[19]; b: the cold seep carbonates exposed from the Marnes Bleues formation in southern France during the late Apudian Period exhibit a significant increase in Ni content within the organic-rich zone[86]; c: microbial mineralization during the methane cycle is believed to have contributed, to some extents, to the formation of a high Ni-grade laterite deposit in Colombia, where the maximum Ni content reaches 8 wt.%[85]; d: methanogenic bacteria have been shown to cause significant Ni isotope fractionation[88]; e: Ni isotopes suggest that the intensification of methanogenesis played a role in partially ending the Snowball Earth (635 million years ago)[92].
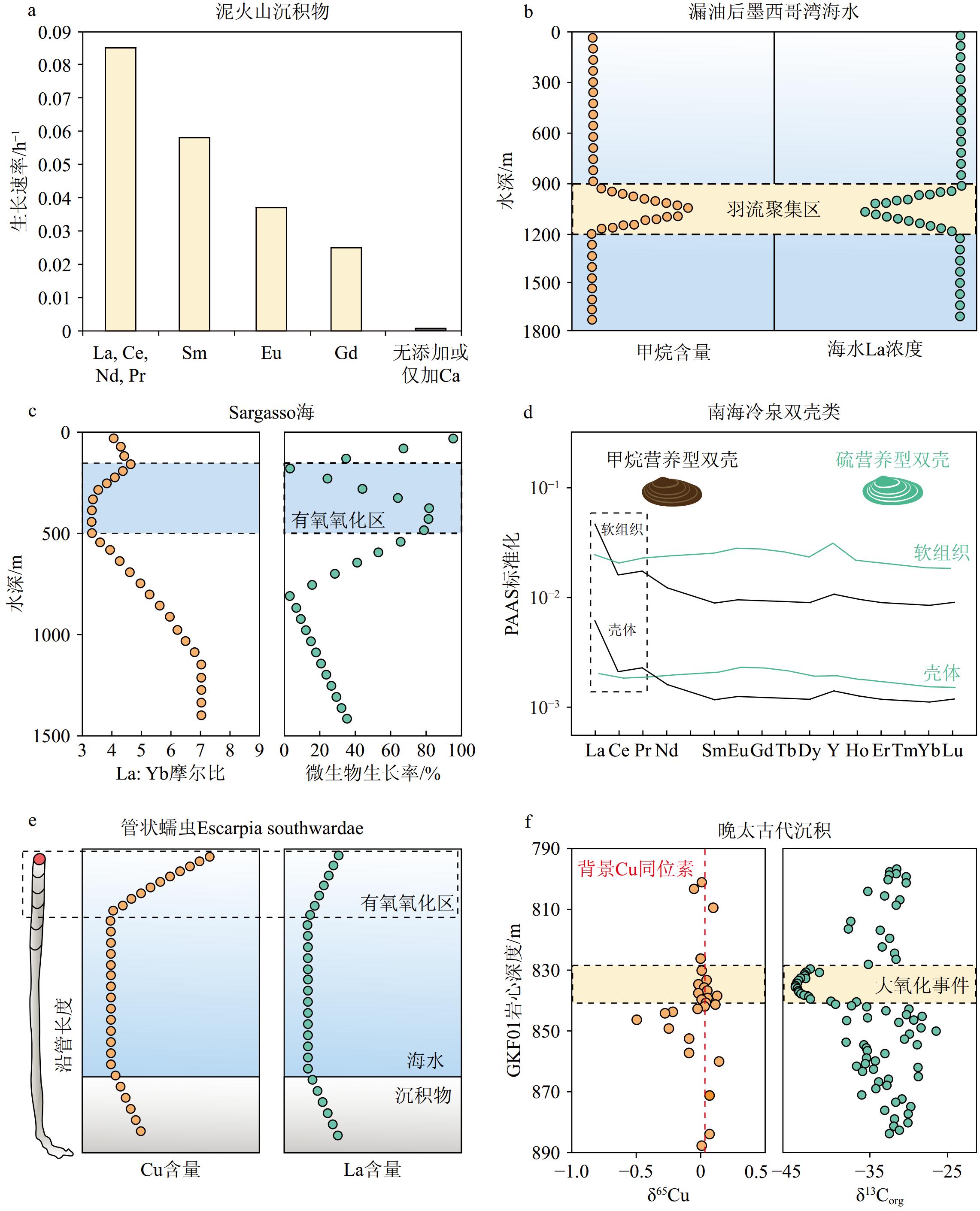
图 5 甲烷有氧氧化作用的野外证据
a: 极端嗜酸性甲烷氧化菌在加入轻REE后生长速率明显加快[20];b: 墨西哥湾漏油事故发生后,在水深900-1200m处形成了碳氢化合物羽流区。该深度范围内海水甲烷浓度明显升高,但轻REE含量明显降低[21];c: 甲烷有氧氧化作用造成了马尾藻海200-500m水深处海水中轻REE含量明显减少[23];d: 甲烷营养型双壳的软组织和钙质壳体都有明显的轻REE富集,但硫营养型双壳则没有此类特征[22];e: 管状蠕虫Escarpia southwardae几丁质外壳的Cu和La含量含量在顶端明显升高[95];f: 在大氧化事件开始之前,AeOM过程就已经不断增强并持续了一段时间,造成晚太古代沉积体系的δ65Cu值明显降低[97]。
Figure 5. Field evidence of aerobic oxidation of methane
a: the growth rate of highly acidophilic methane-oxidizing bacteria is significantly enhanced upon the introduction of light rare earth elements[20]; b: following the oil spill in the Gulf of Mexico, a hydrocarbon plume region developed at a water depth ranging from 900 to 1200 m. In this depth range, there was a noticeable increase in the concentration of methane in seawater, while the abundance of light rare earth elements decreased significantly[21]; c: the aerobic oxidation of methane led to a significant decrease in light rare earth elements in seawater at depths of 200-500m in the Sargasso Sea[23]; d: methanotrophic bivalves exhibit enrichment of light REE in their soft tissues and calcareous shells, whereas thiotrophic bivalves lack this characteristic[22]; e: the chitinous tube of the tubeworm Escarpia southwardae showed a noticeable increase in Cu and La contents at the top[95]; f: prior to the Great Oxidation Event, the aerobic oxidation of methane intensified continuously over a period, resulting in a significant decrease in the δ65Cu value of the late Archean sediments[97].
表 1 涉及甲烷循环过程的反应方程式
Table 1 Reaction equations of methane cycle processes
反应 甲烷循环过程 具体类型 反应方程式 1 产甲烷作用 氢营养型 4H2 + CO2 → CH4 + 2H2O 2 4HCOOH → CH4 + 3CO2 + 2H2O 3 4CO + 2H2O → CH4 + 3CO2 4 乙酸营养型 CH3COOH → CH4 + CO2 5 甲基营养型 CH3OH + H2 → CH4 + H2O 6 4CH3OH→3CH4 + CO2 + 2H2O 7 2(CH3)2-S + 2H2O → 3CH4 + CO2 + 2H2S 8 4CH3-NH2 + 2H2O → 3CH4 + CO2 + 4NH3 9 2(CH3)2-NH + 2H2O → 3CH4 + CO2 + 2NH3 10 4(CH3)-N + 6H2O → 9CH4 + 3CO2 + 4NH3 11 4CH3NH3Cl + 2H2O → 3CH4 + CO2 + 4NH4Cl 12 甲氧基营养型 4CH3-O-R + 2H2O → 3CH4 + CO2 + 4R-OH 13 烷基营养型 4C16H34 + 30H2O → 49CH4 + 15CO2 14 甲烷厌氧氧化 反向产甲烷 CH4 + 2H2O → CO2 + 4H2 15 SO42– + 4H2 + H+ → HS– + 4H2O 16 乙酸生成 2CH4 + 2H2O → CH3COOH + 4H2 17 (同15) SO42– + 4H2 + H+ → HS– + 4H2O 18 CH3COOH + SO42– → 2HCO3– + HS– + H+ 19 CH4 + SO42– → HCO3– + HS– + H2O 20 CH4 + HCO3– → CH3COO– + H2O 21 CH3COO– + SO42– → 2HCO3– + 2HS– 22 (同19) CH4 + SO42– → HCO3– + HS– + H2O 23 甲基生成 3CH4 + HCO3– + 5H+ + 4HS– → CH3-SH + 3H2O 24 4CH3-SH + 3SO42– → 4HCO3– +7HS– + 5H+ 25 甲烷有氧氧化 总反应 CH4 + 2O2 → CO2 + 2H2O 26 甲烷转化为甲醇 CH4 + O2 + 2e– + 2H+ → CH3OH + H2O 27 甲醇转化为甲醛 CH3OH → HCHO + H2 28 甲醛转化为甲酸 HCHO + H2O → HCOOH + H2 29 甲酸转化为CO2和H2 HCOOH → CO2 + H2 -
[1] Reay D S, Smith P, Christensen T R, et al. Methane and global environmental change[J]. Annual Review of Environment and Resources, 2018, 43:165-192. doi: 10.1146/annurev-environ-102017-030154
[2] Mißbach H, Duda J P, van den Kerkhof A M, et al. Ingredients for microbial life preserved in 3.5 billion-year-old fluid inclusions[J]. Nature Communications, 2021, 12(1):1101. doi: 10.1038/s41467-021-21323-z
[3] Chen C S, Qin S F, Wang Y P, et al. High temperature methane emissions from Large Igneous Provinces as contributors to late Permian mass extinctions[J]. Nature Communications, 2022, 13(1):6893. doi: 10.1038/s41467-022-34645-3
[4] Peng S S, Lin X, Thompson R L, et al. Wetland emission and atmospheric sink changes explain methane growth in 2020[J]. Nature, 2022, 612(7940):477-482. doi: 10.1038/s41586-022-05447-w
[5] Rocher-Ros G, Stanley E H, Loken L C, et al. Global methane emissions from rivers and streams[J]. Nature, 2023, 621(7979):530-535. doi: 10.1038/s41586-023-06344-6
[6] Levin L A. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes[M]//Gibson R N, Atkinson R J A, Gordon J D M. Oceanography and Marine Biology: an Annual Review. Boca Raton: CRC Press, 2005: 1-46.
[7] Yang S S, Lv Y X, Liu X P, et al. Genomic and enzymatic evidence of acetogenesis by anaerobic methanotrophic archaea[J]. Nature Communications, 2020, 11(1):3941. doi: 10.1038/s41467-020-17860-8
[8] Giovannelli D. Trace metal availability and the evolution of biogeochemistry[J]. Nature Reviews Earth & Environment, 2023, 4(9):597-598.
[9] Tagliabue A, Bowie A R, Boyd P W, et al. The integral role of iron in ocean biogeochemistry[J]. Nature, 2017, 543(7643):51-59. doi: 10.1038/nature21058
[10] Swanner E D, Planavsky N J, Lalonde S V, et al. Cobalt and marine redox evolution[J]. Earth and Planetary Science Letters, 2014, 390:253-263. doi: 10.1016/j.jpgl.2014.01.001
[11] Shafiee R T, Diver P J, Snow J T, et al. Marine ammonia-oxidising archaea and bacteria occupy distinct iron and copper niches[J]. ISME Communications, 2021, 1(1):1. doi: 10.1038/s43705-021-00001-7
[12] Buessecker S, Palmer M, Lai D X, et al. An essential role for tungsten in the ecology and evolution of a previously uncultivated lineage of anaerobic, thermophilic Archaea[J]. Nature Communications, 2022, 13(1):3773. doi: 10.1038/s41467-022-31452-8
[13] Dupont C L, Butcher A, Valas R E, et al. History of biological metal utilization inferred through phylogenomic analysis of protein structures[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(23):10567-10572.
[14] Mele B H, Monticelli M, Leone S, et al. Oxidoreductases and metal cofactors in the functioning of the earth[J]. Essays in Biochemistry, 2023, 67(4):653-670. doi: 10.1042/EBC20230012
[15] Rudroff F, Mihovilovic M D, Gröger H, et al. Opportunities and challenges for combining chemo- and biocatalysis[J]. Nature Catalysis, 2018, 1(1):12-22. doi: 10.1038/s41929-017-0010-4
[16] Glass J B, Orphan V J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide[J]. Frontiers in Microbiology, 2012, 3:61.
[17] Glass J B, Yu H, Steele J A, et al. Geochemical, metagenomic and metaproteomic insights into trace metal utilization by methane-oxidizing microbial consortia in sulphidic marine sediments[J]. Environmental Microbiology, 2014, 16(6):1592-1611. doi: 10.1111/1462-2920.12314
[18] Glass J B, Chen S, Dawson K S, et al. Trace metal imaging of sulfate-reducing bacteria and methanogenic archaea at single-cell resolution by synchrotron X-ray fluorescence imaging[J]. Geomicrobiology Journal, 2018, 35(1):81-89. doi: 10.1080/01490451.2017.1321068
[19] Krüger M, Meyerdierks A, Glöckner F O, et al. A conspicuous nickel protein in microbial mats that oxidize methane anaerobically[J]. Nature, 2003, 426(6968):878-881. doi: 10.1038/nature02207
[20] Pol A, Barends T R M, Dietl A, et al. Rare earth metals are essential for methanotrophic life in volcanic mudpots[J]. Environmental Microbiology, 2014, 16(1):255-264. doi: 10.1111/1462-2920.12249
[21] Shiller A M, Chan E W, Joung D J, et al. Light rare earth element depletion during Deepwater Horizon blowout methanotrophy[J]. Scientific Reports, 2017, 7(1):10389. doi: 10.1038/s41598-017-11060-z
[22] Wang X, Barrat J A, Bayon G, et al. Lanthanum anomalies as fingerprints of methanotrophy[J]. Geochemical Perspectives Letters, 2020, 14:26-30. doi: 10.7185/geochemlet.2019
[23] Meyer A C S, Grundle D, Cullen J T. Selective uptake of rare earth elements in marine systems as an indicator of and control on aerobic bacterial methanotrophy[J]. Earth and Planetary Science Letters, 2021, 558:116756. doi: 10.1016/j.jpgl.2021.116756
[24] Garcia P S, Gribaldo S, Borrel G. Diversity and evolution of methane-related pathways in archaea[J]. Annual Review of Microbiology, 2022, 76:727-755. doi: 10.1146/annurev-micro-041020-024935
[25] Mayumi D, Mochimaru H, Tamaki H, et al. Methane production from coal by a single methanogen[J]. Science, 2016, 354(6309):222-225. doi: 10.1126/science.aaf8821
[26] Wang Y Z, Wegener G, Williams T A, et al. A methylotrophic origin of methanogenesis and early divergence of anaerobic multicarbon alkane metabolism[J]. Science Advances, 2021, 7(27):eabj1453. doi: 10.1126/sciadv.abj1453
[27] Zhou Z, Zhang C J, Liu P F, et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species[J]. Nature, 2022, 601(7892):257-262. doi: 10.1038/s41586-021-04235-2
[28] Thauer R K, Kaster A K, Seedorf H, et al. Methanogenic archaea: ecologically relevant differences in energy conservation[J]. Nature Reviews Microbiology, 2008, 6(8):579-591. doi: 10.1038/nrmicro1931
[29] Liu Y C, Whitman W B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea[J]. Annals of the New York Academy of Sciences, 2008, 1125(1):171-189. doi: 10.1196/annals.1419.019
[30] Fricke W F, Seedorf H, Henne A, et al. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis[J]. Journal of Bacteriology, 2006, 188(2):642-658. doi: 10.1128/JB.188.2.642-658.2006
[31] Thauer R K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson: 1998 Marjory Stephenson Prize Lecture[J]. Microbiology, 1998, 144(9):2377-2406. doi: 10.1099/00221287-144-9-2377
[32] Ferry J G. Enzymology of one-carbon metabolism in methanogenic pathways[J]. FEMS Microbiology Reviews, 1999, 23(1):13-38. doi: 10.1111/j.1574-6976.1999.tb00390.x
[33] Zhuang G C, Elling F J, Nigro L M, et al. Multiple evidence for methylotrophic methanogenesis as the dominant methanogenic pathway in hypersaline sediments from the Orca Basin, Gulf of Mexico[J]. Geochimica et Cosmochimica Acta, 2016, 187:1-20. doi: 10.1016/j.gca.2016.05.005
[34] Kurth J M, Nobu M K, Tamaki H, et al. Methanogenic archaea use a bacteria-like methyltransferase system to demethoxylate aromatic compounds[J]. The ISME Journal, 2021, 15(12):3549-3565. doi: 10.1038/s41396-021-01025-6
[35] Laso-Pérez R, Hahn C, van Vliet D M, et al. Anaerobic degradation of non-methane alkanes by “Candidatus Methanoliparia” in hydrocarbon seeps of the Gulf of Mexico[J]. mBio, 2019, 10(4):e01814-19.
[36] Zerkle A L, House C H, Brantley S L. Biogeochemical signatures through time as inferred from whole microbial genomes[J]. American Journal of Science, 2005, 305(6-8):467-502. doi: 10.2475/ajs.305.6-8.467
[37] Timmis K N. Handbook of Hydrocarbon and Lipid Microbiology[M]. Berlin Heidelberg: Springer, 2010.
[38] Hedderich R, Whitman W B. Physiology and biochemistry of the methane-producing archaea[M]//Dworkin M, Falkow S, Rosenberg E, et al. The Prokaryotes. New York: Springer, 2006: 1050-1079.
[39] Wagner T, Koch J, Ermler U, et al. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction[J]. Science, 2017, 357(6352):699-703. doi: 10.1126/science.aan0425
[40] Segarra K E A, Schubotz F, Samarkin V, et al. High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions[J]. Nature Communications, 2015, 6(1):7477. doi: 10.1038/ncomms8477
[41] Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process[J]. Annual Review of Microbiology, 2009, 63:311-334. doi: 10.1146/annurev.micro.61.080706.093130
[42] Hoehler T M, Alperin M J, Albert D B, et al. Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium[J]. Global Biogeochemical Cycles, 1994, 8(4):451-463. doi: 10.1029/94GB01800
[43] Hallam S J, Putnam N, Preston C M, et al. Reverse methanogenesis: testing the hypothesis with environmental genomics[J]. Science, 2004, 305(5689):1457-1462. doi: 10.1126/science.1100025
[44] Scheller S, Goenrich M, Boecher R, et al. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane[J]. Nature, 2010, 465(7298):606-608. doi: 10.1038/nature09015
[45] Valentine D L, Reeburgh W S. New perspectives on anaerobic methane oxidation[J]. Environmental Microbiology, 2000, 2(5):477-484. doi: 10.1046/j.1462-2920.2000.00135.x
[46] Lessner D J, Li L Y, Li Q B, et al. An unconventional pathway for reduction of CO2 to methane in CO-grown Methanosarcina acetivorans revealed by proteomics[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(47):17921-17926.
[47] Zehnder A J, Brock T D. Methane formation and methane oxidation by methanogenic bacteria[J]. Journal of Bacteriology, 1979, 137(1):420-432. doi: 10.1128/jb.137.1.420-432.1979
[48] Moran J J, Beal E J, Vrentas J M, et al. Methyl sulfides as intermediates in the anaerobic oxidation of methane[J]. Environmental Microbiology, 2008, 10(1):162-173. doi: 10.1111/j.1462-2920.2007.01441.x
[49] Milucka J, Ferdelman T G, Polerecky L, et al. Zero-valent sulphur is a key intermediate in marine methane oxidation[J]. Nature, 2012, 491(7425):541-546. doi: 10.1038/nature11656
[50] Jermy A. Zero-valent sulphur and marine methane oxidation[J]. Nature Reviews Microbiology, 2013, 11(1):5.
[51] Lichtschlag A, Kamyshny A, Ferdelman T G, et al. Intermediate sulfur oxidation state compounds in the euxinic surface sediments of the Dvurechenskii mud volcano (Black Sea)[J]. Geochimica et Cosmochimica Acta, 2013, 105:130-145. doi: 10.1016/j.gca.2012.11.025
[52] Zhang X, Du Z F, Zheng R E, et al. Development of a new deep-sea hybrid Raman insertion probe and its application to the geochemistry of hydrothermal vent and cold seep fluids[J]. Deep Sea Research Part I: Oceanographic Research Papers, 2017, 123:1-12. doi: 10.1016/j.dsr.2017.02.005
[53] Zopfi J, Ferdelman T G, Fossing H. Distribution and fate of sulfur intermediates – sulfite, tetrathionate, thiosulfate, and elemental sulfur – in marine sediments[M]//Amend J P, Edwards K J, Lyons T W. Sulfur Biogeochemistry – Past and Present. Boulder, Colorado: Geological Society of America, 2004: 97-116.
[54] Zhang J, Liu R, Xi S C, et al. A novel bacterial thiosulfate oxidation pathway provides a new clue about the formation of zero-valent sulfur in deep sea[J]. The ISME Journal, 2020, 14(9):2261-2274. doi: 10.1038/s41396-020-0684-5
[55] Liu R, Shan Y Q, Xi S C, et al. A deep-sea sulfate-reducing bacterium generates zero-valent sulfur via metabolizing thiosulfate[J]. mLife, 2022, 1(3):257-271. doi: 10.1002/mlf2.12038
[56] McGlynn S E, Chadwick G L, Kempes C P, et al. Single cell activity reveals direct electron transfer in methanotrophic consortia[J]. Nature, 2015, 526(7574):531-535. doi: 10.1038/nature15512
[57] Wegener G, Krukenberg V, Riedel D, et al. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria[J]. Nature, 2015, 526(7574):587-590. doi: 10.1038/nature15733
[58] Scheller S, Yu H, Chadwick G L, et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction[J]. Science, 2016, 351(6274):703-707. doi: 10.1126/science.aad7154
[59] Dekas A E, Poretsky R S, Orphan V J. Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia[J]. Science, 2009, 326(5951):422-426. doi: 10.1126/science.1178223
[60] Thauer R K. Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2[J]. Current Opinion in Microbiology, 2011, 14(3):292-299. doi: 10.1016/j.mib.2011.03.003
[61] Mao S H, Zhang H H, Zhuang G C, et al. Aerobic oxidation of methane significantly reduces global diffusive methane emissions from shallow marine waters[J]. Nature Communications, 2022, 13(1):7309. doi: 10.1038/s41467-022-35082-y
[62] Chen Y, Murrell J C. Ecology of aerobic methanotrophs and their role in methane cycling[M]//Timmis K N. Handbook of Hydrocarbon and Lipid Microbiology. Berlin Heidelberg: Springer, 2010: 3067-3076.
[63] Valentine D L, Blanton D C, Reeburgh W S, et al. Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel river Basin[J]. Geochimica et Cosmochimica Acta, 2001, 65(16):2633-2640. doi: 10.1016/S0016-7037(01)00625-1
[64] Blumenberg M, Seifert R, Michaelis W. Aerobic methanotrophy in the oxic–anoxic transition zone of the Black Sea water column[J]. Organic Geochemistry, 2007, 38(1):84-91. doi: 10.1016/j.orggeochem.2006.08.011
[65] Leonte M, Kessler J D, Kellermann M Y, et al. Rapid rates of aerobic methane oxidation at the feather edge of gas hydrate stability in the waters of Hudson Canyon, US Atlantic Margin[J]. Geochimica et Cosmochimica Acta, 2017, 204:375-387. doi: 10.1016/j.gca.2017.01.009
[66] Boetius A, Wenzhöfer F. Seafloor oxygen consumption fuelled by methane from cold seeps[J]. Nature Geoscience, 2013, 6(9):725-734. doi: 10.1038/ngeo1926
[67] Spencer-Jones C L, Wagner T, Talbot H M. A record of aerobic methane oxidation in tropical Africa over the last 2.5Ma[J]. Geochimica et Cosmochimica Acta, 2017, 218:27-39. doi: 10.1016/j.gca.2017.08.042
[68] Anthony K W, Daanen R, Anthony P, et al. Methane emissions proportional to permafrost carbon thawed in Arctic lakes since the 1950s[J]. Nature Geoscience, 2016, 9(9):679-682. doi: 10.1038/ngeo2795
[69] Weber T, Wiseman N A, Kock A. Global ocean methane emissions dominated by shallow coastal waters[J]. Nature Communications, 2019, 10(1):4584. doi: 10.1038/s41467-019-12541-7
[70] Kalyuzhnaya M G, Puri A W, Lidstrom M E. Metabolic engineering in methanotrophic bacteria[J]. Metabolic Engineering, 2015, 29:142-152. doi: 10.1016/j.ymben.2015.03.010
[71] Stanley S H, Prior S D, Leak D J, et al. Copper stress underlies the fundamental change in intracellular location of methane mono-oxygenase in methane-oxidizing organisms: studies in batch and continuous cultures[J]. Biotechnology Letters, 1983, 5(7):487-492. doi: 10.1007/BF00132233
[72] Picone N, Op den Camp H J M. Role of rare earth elements in methanol oxidation[J]. Current Opinion in Chemical Biology, 2019, 49:39-44. doi: 10.1016/j.cbpa.2018.09.019
[73] Ōgushi S, Ando M, Tsuru D. Formaldehyde dehydrogenase from Pseudomonas putida: a zinc metalloenzyme[J]. The Journal of Biochemistry, 1984, 96(5):1587-1591. doi: 10.1093/oxfordjournals.jbchem.a134988
[74] 朱小飞, 谭相石. 金属组学: Wood-Ljungdahl通路中的金属蛋白/金属酶[J]. 中国科学 B辑: 化学, 2009, 39(7): 607-619 ZHU Xiaofei, TAN Xiangshi. Metalloproteins/metalloenzymes for the synthesis of acetyl-CoA in the Wood-ljungdahl pathway[J]. Science in China Series B: Chemistry, 2009, 52(12): 2071-2082.]
[75] Smrzka D, Feng D, Himmler T, et al. Trace elements in methane-seep carbonates: potentials, limitations, and perspectives[J]. Earth-Science Reviews, 2020, 208:103263. doi: 10.1016/j.earscirev.2020.103263
[76] Feng D, Lin Z J, Bian Y Y, et al. Rare earth elements of seep carbonates: indication for redox variations and microbiological processes at modern seep sites[J]. Journal of Asian Earth Sciences, 2013, 65:27-33. doi: 10.1016/j.jseaes.2012.09.002
[77] Chen L Y, Jin M, Wang X D, et al. The effects of diagenetic processes and fluid migration on rare earth element and organic matter distribution in seep-related sediments: a case study from the South China Sea[J]. Journal of Asian Earth Sciences, 2020, 191:104233. doi: 10.1016/j.jseaes.2020.104233
[78] Wang X D, Bayon G, Kim J H, et al. Trace element systematics in cold seep carbonates and associated lipid compounds[J]. Chemical Geology, 2019, 528:119277. doi: 10.1016/j.chemgeo.2019.119277
[79] Lee D H, Kim J H, Lee Y M, et al. Metalloenzyme signatures in authigenic carbonates from the Chukchi Borderlands in the western Arctic Ocean[J]. Scientific Reports, 2022, 12(1):16597. doi: 10.1038/s41598-022-21184-6
[80] Shima S, Thauer R K. Methyl-coenzyme M reductase and the anaerobic oxidation of methane in methanotrophic Archaea[J]. Current Opinion in Microbiology, 2005, 8(6):643-648. doi: 10.1016/j.mib.2005.10.002
[81] Wrede C, Krukenberg V, Dreier A, et al. Detection of metabolic key enzymes of methane turnover processes in cold seep microbial biofilms[J]. Geomicrobiology Journal, 2013, 30(3):214-227. doi: 10.1080/01490451.2012.665150
[82] Mayr S, Latkoczy C, Krüger M, et al. Structure of an F430 variant from archaea associated with anaerobic oxidation of methane[J]. Journal of the American Chemical Society, 2008, 130(32):10758-10767. doi: 10.1021/ja802929z
[83] Shima S, Krueger M, Weinert T, et al. Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically[J]. Nature, 2012, 481(7379):98-101. doi: 10.1038/nature10663
[84] Allen K D, Wegener G, White R H. Discovery of multiple modified F430 coenzymes in methanogens and anaerobic methanotrophic archaea suggests possible new roles for F430 in nature[J]. Applied and Environmental Microbiology, 2014, 80(20):6403-6412. doi: 10.1128/AEM.02202-14
[85] Castrillón Peña A, Cramer T H, Guerrero J. Hydrothermal organic aggregates associated with the High-Ni grades of the Cerro Matoso laterite deposit, Montelibano, Colombia[J]. Ofioliti, 2022, 47(2):113-135.
[86] Reitner J, Blumenberg M, Walliser E O, et al. Methane-derived carbonate conduits from the late Aptian of Salinac (Marne Bleues, Vocontian Basin, France): petrology and biosignatures[J]. Marine and Petroleum Geology, 2015, 66:641-652. doi: 10.1016/j.marpetgeo.2015.05.029
[87] Hausrath E M, Liermann L J, House C H, et al. The effect of methanogen growth on mineral substrates: will Ni markers of methanogen-based communities be detectable in the rock record?[J]. Geobiology, 2007, 5(1):49-61. doi: 10.1111/j.1472-4669.2007.00095.x
[88] Cameron V, Vance D, Archer C, et al. A biomarker based on the stable isotopes of nickel[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(27):10944-10948.
[89] Chen C, Wang J S, Algeo T J, et al. Sulfate-driven anaerobic oxidation of methane inferred from trace-element chemistry and nickel isotopes of pyrite[J]. Geochimica et Cosmochimica Acta, 2023, 349:81-95. doi: 10.1016/j.gca.2023.04.002
[90] Konhauser K O, Pecoits E, Lalonde S V, et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event[J]. Nature, 2009, 458(7239):750-753. doi: 10.1038/nature07858
[91] Fontecilla-Camps J C. Nickel and the origin and early evolution of life[J]. Metallomics, 2022, 14(4):mfac016. doi: 10.1093/mtomcs/mfac016
[92] Zhao Z Q, Shen B, Zhu J M, et al. Active methanogenesis during the melting of Marinoan snowball Earth[J]. Nature Communications, 2021, 12(1):955. doi: 10.1038/s41467-021-21114-6
[93] Valentine D L. Emerging topics in marine methane biogeochemistry[J]. Annual Review of Marine Science, 2011, 3:147-171. doi: 10.1146/annurev-marine-120709-142734
[94] Durisch-Kaiser E, Klauser L, Wehrli B, et al. Evidence of intense archaeal and bacterial methanotrophic activity in the black sea water column[J]. Applied and Environmental Microbiology, 2005, 71(12):8099-8106. doi: 10.1128/AEM.71.12.8099-8106.2005
[95] Bayon G, Lemaitre N, Barrat J A, et al. Microbial utilization of rare earth elements at cold seeps related to aerobic methane oxidation[J]. Chemical Geology, 2020, 555:119832. doi: 10.1016/j.chemgeo.2020.119832
[96] Guggenheim C, Brand A, Bürgmann H, et al. Aerobic methane oxidation under copper scarcity in a stratified lake[J]. Scientific Reports, 2019, 9(1):4817. doi: 10.1038/s41598-019-40642-2
[97] Zavina-James N A V, Zerkle A L, Steele R C J, et al. A copper isotope investigation of methane cycling in Late Archaean sediments[J]. Precambrian Research, 2021, 362:106267. doi: 10.1016/j.precamres.2021.106267



 下载:
下载: