Advances in the study of methane-metabolizing microbial communities in marine sediments
-
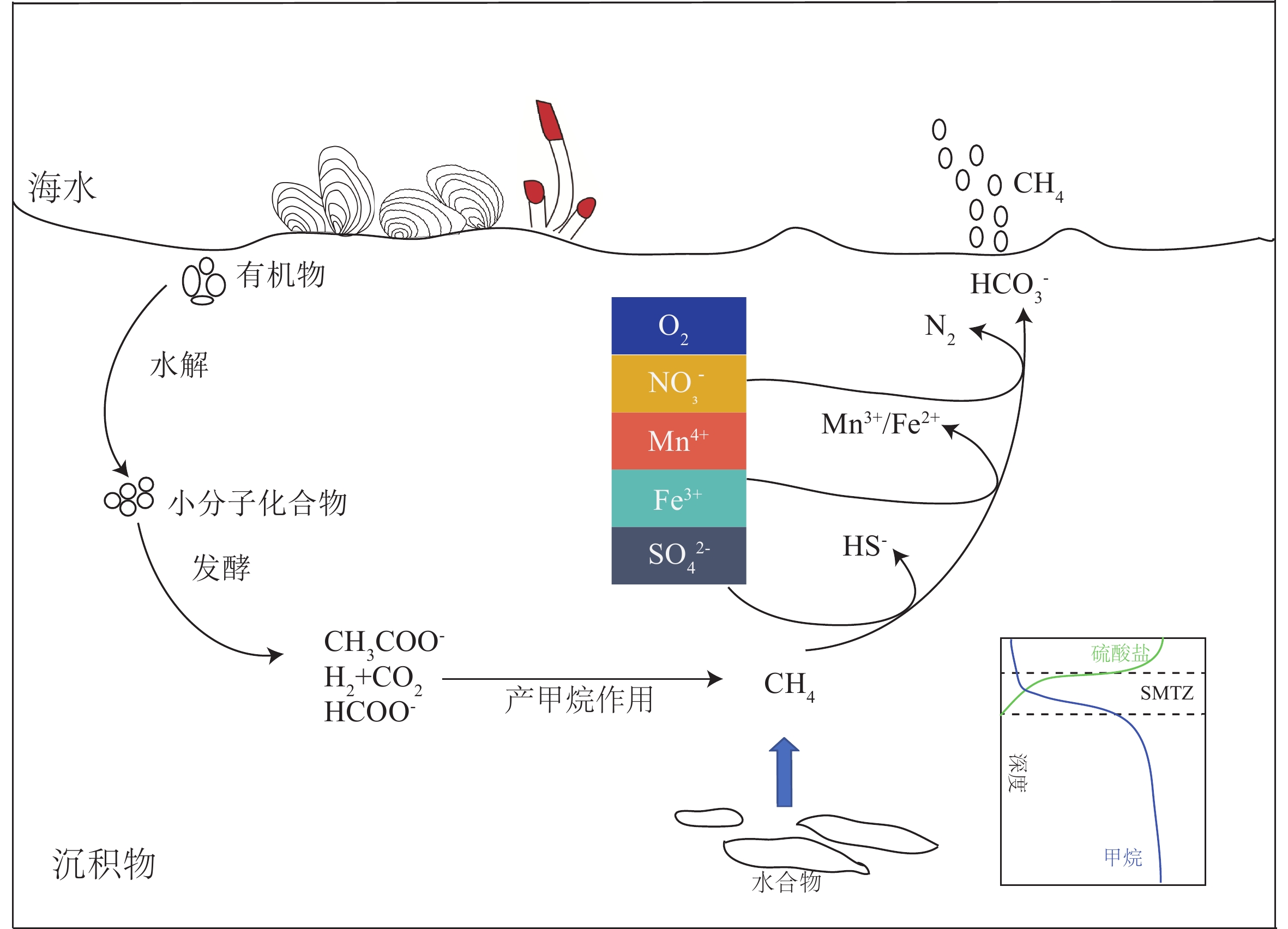
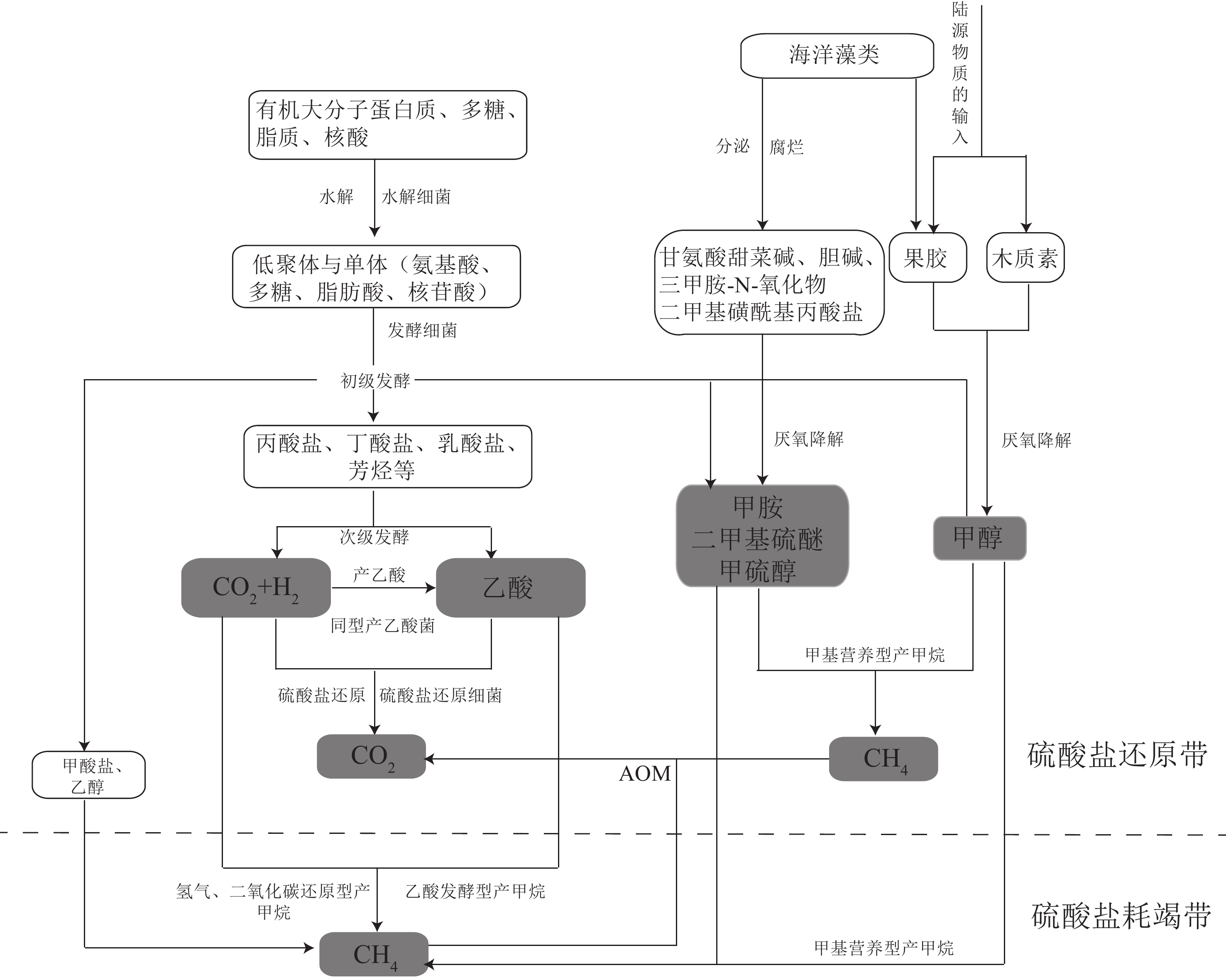
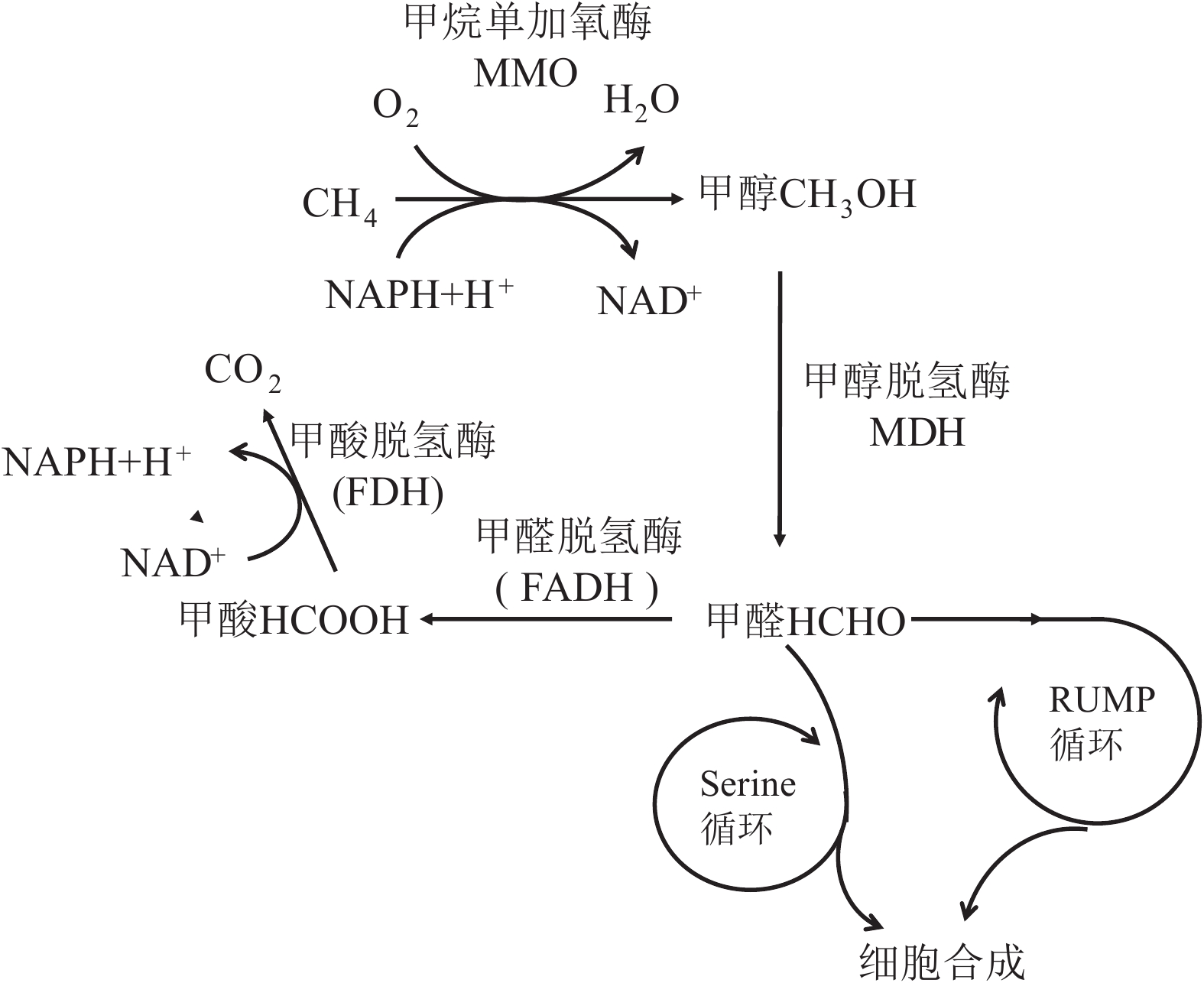
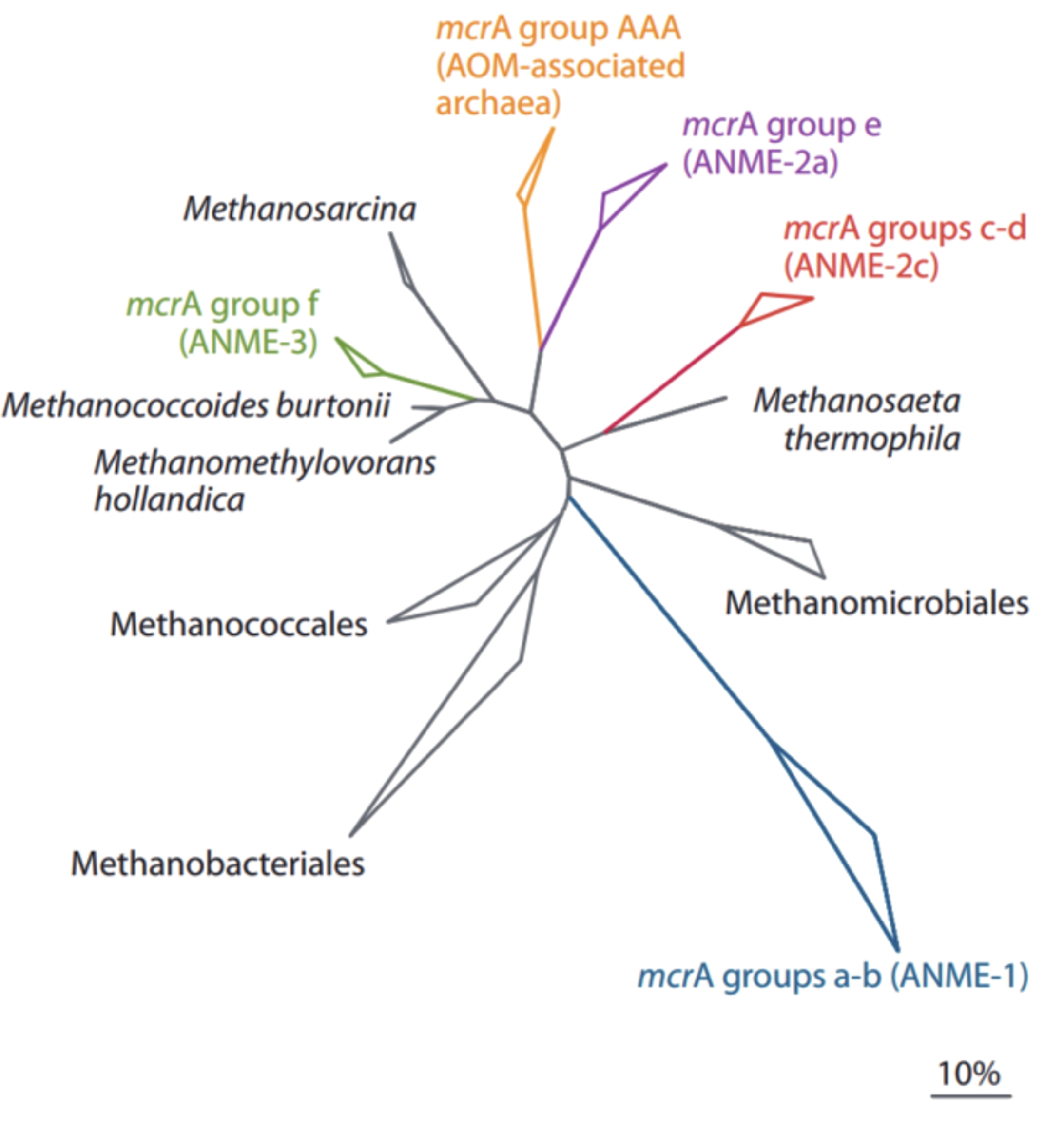
摘要: 甲烷是一种重要的温室气体,深刻影响着全球的气候变化。同时,甲烷还是海底潜在能源—天然气水合物的主要成分。海洋沉积物是甲烷生物转化的一个重要生态区域,产甲烷菌主要利用H2、CO2及简单的有机物(甲醇、甲胺、二甲基硫等)作为底物生成甲烷,产生的甲烷在向上迁移的过程中主要被甲烷厌氧氧化(anaerobic oxidation of methane, AOM)和甲烷好氧氧化(aerobic oxidation of methane,AeOM)消耗,进而大大减少了甲烷向大气的排放量。AeOM主要发生在含氧的沉积物及沉积物-水界面中,由甲烷好氧氧化菌(aerobic methane-oxidizing bacteria, MOB)介导。然而,绝大部分甲烷在穿透缺氧沉积物层之前是被AOM反应消耗,甲烷厌氧氧化古菌(anaerobic methanotrophic archaea,ANME)是主要的参与者,这些功能微生物耦联电子受体SO42−、NO2−/NO3−或Fe3+和Mn4+将甲烷进行氧化。本文对产甲烷菌和甲烷氧化菌的种类、代谢途径及其在海洋沉积物中的分布特征进行了综述,并在前人工作基础上,对今后海洋生境中甲烷代谢过程的研究进行了展望,以期为进一步开展海洋环境中甲烷的生物转化过程及元素耦合的研究提供理论依据。Abstract: Methane is an important greenhouse gas affecting the global climate. Meanwhile, methane is a major component of natural gas hydrate which regarded as a potential energy resource below seafloor. Seafloor sediment is an important ecological region for methane biotransformation. The methanogens can use H2, CO2, and simple organic compounds (e.g. methanol, methylamines, dimethylsulfide) as substrates to produce methane. The methane produced in the bottom of the sediments would be consumed by aerobic methanotrophs and anaerobic methanotrophs during its upward migration, which reduces greatly the methane emissions to the atmosphere. Aerobic methane oxidation occurs mainly in oxygenated sediments and sediment-water interfaces, and is mediated by aerobic methane-oxidizing bacteria. However, most of the methane is consumed by anaerobic methane oxidation before it reaches the seafloor. The anaerobic methanotrophs oxidize methane coupled by SO42−, NO2−/NO3− or Fe3+/Mn4+. We reviewed the status quo and perspectives of the taxonomy, metabolic and ecological diversity of methanogens and methanotrophs in marine sediments, and emphasized deficiencies and issues need to be solved in future studies. This review provided theoretical foundation for the study of biotransformation process and element coupling of methane in marine environment.
-
Keywords:
- methane /
- marine sediment /
- methanogen /
- methanotrophs
-
东亚大陆边缘河流沉积物源汇过程是地球科学领域的重大科学问题[1],中国东部海域浅海陆架沉积地层记录了该大型源汇体系的演化过程,是重建新生代亚洲大陆构造隆升、季风演化以及气候变化等过程的重要信息载体[2-3],同时,浅海陆架沉积记录对全球海平面变化也具有重要指示[4-6]。
黄海是一个典型的半封闭型陆架海,周边河流主要包括中国大陆的长江、淮河、黄河以及朝鲜半岛汉江、锦江等河流,接受上述河流的大量陆源碎屑物质[7]。受黄海暖流、沿岸流等海流以及冷水团的控制,在北黄海西部、南黄海中部、济州岛西南和南黄海东南部形成了多个泥质区[8]。其中,南黄海中部泥质区的研究成果最为丰富。在物源研究方面,利用矿物学和地球化学方面的证据,多数学者认为南黄海中部泥质区沉积物主要由黄河和长江供应[7,9-12]。其他潜在来源是局部小河流,如淮河等,其年入海沉积物通量(约76 Mt/a)相对较低,对中部泥质区的贡献可能很小[13]。发源于朝鲜半岛的主要河流,如汉江、锦江等,向黄海东部输送沉积物通量仅为18 Mt/a[13],对中部泥质区沉积物贡献微不足道[7]。此外,由于黄河和长江巨量泥沙的稀释,风尘对南黄海沉积的影响几乎可忽略不计[14]。利用钻孔沉积物资料,Yao等人研究表明,南黄海0.88 Ma 以来物质来源主要受黄河控制[15]。CSDP-1孔的碎屑锆石U-Pb年龄分析显示0.78 Ma中部泥质区才开始有黄河源的物质,并且自此黄河成为钻孔区主要物质来源[16]。
多数物源研究成果以细粒沉积物为研究对象,主要分析了末次间冰期以来短时间尺度的物源变化。虽然已经形成南黄海中部泥质区为黄河源和长江源物质混合区的认识,但二者占主导的区域分界还不够清晰,地质历史时期不同河流对南黄海海底沉积物影响程度的认识还存在较多分歧。
锆石具有很强的抗风化能力,广泛分布于河流、湖泊和三角洲各种沉积环境的陆源碎屑沉积物中。通过多个样品的年龄图谱相互比较,并分析整个流域的构造运动历史及沉积环境特征,可以很好地追踪沉积物的输运轨迹[17-18]。在过去20年里,单颗粒矿物原位分析技术的发展使得碎屑锆石U-Pb年代学方法成为沉积物物源研究的标准方法之一[19-21]。本文以南黄海中部泥质区南部及泥质区外缘4个表层沉积物样品和5个钻孔沉积物样品的碎屑锆石U-Pb年龄进行分析,为南黄海中部泥质区物源分界、地质历史时期物源及沉积环境演化研究提供新证据。
1. 地质背景
南黄海位于中国大陆和朝鲜半岛之间(图1),平均水深46 m,最深处位于济州岛北侧,深达140 m[22]。南黄海海底包括鲁南岸坡及海州湾阶地平原、苏北岸外舌状台地、中部平原、黄海槽洼地、朝鲜半岛岸外台地和济州岛西部砂脊六大地形单元。黄海槽洼地位于南黄海中部,靠近朝鲜半岛一侧,北浅南深,海槽地形东陡西缓[22],最深处在100 m以上。前人研究认为,黄海槽由末次冰期水流运动形成,为全新世海水入侵的主要通道[23]。
南黄海现代海洋流系主要包括黄海暖流、沿岸流等。南黄海中部的黄海冷水团是一个低能环境,为泥质沉积分布区;在南黄海的东部、西南部海区则发育强潮流,形成了潮流沙脊[9]。通过对南黄海打穿第四系的CSDP-2孔研究,发现南黄海在1.66 Ma左右出现第一次海侵,l.66~0.83 Ma南黄海地区以河流相沉积为主,有3次较弱的海侵,直到0.83 Ma以来海侵强度才与现今接近[9,26]。浙闽隆起的进一步沉降使得南黄海中部隆起区在间冰期高海平面时期的海洋环境基本接近现今环境。南黄海西部陆架在MIS5发育范围比现今更广[9]。
南黄海周边河流主要有中国大陆一侧的黄河、长江和朝鲜半岛一侧的汉江、锦江、蟾津江等河流。长江是我国第一长河,流域纬向跨度大,自西向东流经昌都地块、松潘-甘孜褶皱带、秦岭-大别构造带、扬子地块和华夏地块[27],地层出露复杂,元古界至第四系均有分布,包括大面积的碳酸盐岩、陆源碎屑岩和中酸性侵入岩、片岩和片麻岩等[28]。通过大量碎屑锆石U-Pb年龄分析,发现长江碎屑沉积物主要有6组峰:<65、200~300、400~550、700~1000、1800~2000和2400~2600 Ma,其中200~300和700~1000 Ma为主要的两组峰[29]。黄河是我国第二长河,以年输沙量巨大著称,流经松潘-甘孜造山带、秦岭造山带、祁连造山带和华北陆块等多个构造单元[30],碎屑锆石U-Pb年龄结果显示,黄河碎屑沉积物主要有6组峰:200~350、350~500、700~1000、1000~1800、1800~2000和2000~2600 Ma,其中200~350、350~500、1800~2000和2000~2600 Ma为主要的四组峰[31-32]。朝鲜半岛一侧的河流主要为汉江、锦江、蟾津江等中小河流。锆石U-Pb年龄谱显示半岛东西两侧差异明显,年龄范围从早新生代到晚太古代,西部以古生代至新元古代锆石为主,东部以古元古代锆石为主[33]。
2. 材料及方法
2.1 样品
2018年4—5月,青岛海洋地质研究所在南黄海泥质区附近获取表层样300个站位,每个站位用箱式取样器取海底表层20 cm以内样品2袋。SYS90-1A孔由青岛海洋地质研究所在2017年6月利用“明源1001”轮实施,地理坐标为33°48′49″N、123°43′58″E,水深约69.3 m,钻孔深90.1 m,有效样品86管,岩芯总长83.8 m,平均取芯率93.0%。
对SYS90-1A孔全孔进行了连续低场磁化率测量和古地磁交变场退磁测定,结合钻孔上部AMS14C和光释光测年结果,建立了钻孔的年代框架①(图2)。SYS90-1A孔底界年龄约为1.0 Ma,布容正极性时和松山负极性时倒转边界(0.78 Ma)大约位于73.78 m。上部年龄数据较多(图2),根据沉积速率推测晚更新世底界埋深为21.50 m。上部15 m沉积物记录了MIS4、MIS3早期和MIS1的沉积,缺失MIS3晚期至MIS2早期的沉积。
2.2 测试方法
根据岩芯描述及沉积学分析,结合年代学结果,对SYS90-1A孔进行碎屑锆石U-Pb定年分析,共选取5个样品。钻孔岩芯在69.48~69.74 m层位见风化严重的贝壳碎屑,岩性变化明显(图2),推测彼时沉积环境可能发生改变。在层位上下各取1个样品,根据泥质区分布,共选取4个表层沉积物样品进行碎屑锆石U-Pb定年分析。9个沉积物样品位置见图1,埋深和岩性等信息见表1,锆石挑选、制靶、反射光、透射光和阴极荧光(CL)拍摄和测试均在南京宏创地质勘查技术服务有限公司完成。
表 1 沉积物样品位置及岩性信息Table 1. Samples location and lithology information序号 样品编号 位置 埋深/m 岩性 备注 1 SYB80 33°27′15″N、 123°17′46″E 0.20 粉砂 表层样 2 SYB86 33°05′40″N、124°24′17″E 0.20 粉砂 表层样 3 SYB198 33°05′40″N、124°44′02″E 0.20 粉砂 表层样 4 SYB256 33°05′40″N、124°03′48″E 0.20 粉砂 表层样 5 SYS90-1A-B709 33°48′49″N、 123°43′58″E 34.88 粉砂 钻孔样 6 SYS90-1A-C717 33°48′49″N、 123°43′58″E 56.20 细砂 钻孔样 7 SYS90-1A-D235 33°48′49″N、 123°43′58″E 68.88 粉砂 钻孔样 8 SYS90-1A-D275 33°48′49″N、123°43′58″E 69.68 粉砂 钻孔样 9 SYS90-1A-D945 33°48′49″N、123°43′58″E 85.66 粉砂 钻孔样 首先利用常规的浮选和电磁法分离出锆石,随机选出250粒进行环氧树脂制靶,并拍摄显微镜照相(透射光与反射光),然后在场发射扫描电镜实验室进行阴极荧光拍摄,发射扫描电镜型号为TESCAN MIRA3,探头由TESCAN公司提供。
锆石U-Pb定年使用激光剥蚀-电感耦合等离子体质谱仪(LA-ICPMS)完成。激光剥蚀平台采用Resolution SE型193 nm深紫外激光剥蚀进样系统(Applied Spectra,美国),配备S155型双体积样品池。质谱仪采用Agilent7900型电感耦合等离子体质谱仪(Agilent,美国)。详细的调谐参数见Thompson等的文献[35],采用束斑直径50 μm、剥蚀频率10 Hz、能量密度3.5 J/cm2、扫描速度3 μm/s的激光参数剥蚀玻璃标样NIST 612,调节气流以获得高的信号强度。选用100 μm束斑线扫玻璃标样NIST 610对待测元素进行P/A调谐。测量质量数29Si、31P、45Sc、49Ti、56Fe、89Y、91Zr、93Nb、139La、140Ce、141Pr、146Nd、147Sm、151Eu、157Gd、159Tb、163Dy、165Ho、166Er、169Tm、173Yb、175Lu、178Hf、181Ta、202Hg、204Pb、206Pb、207Pb、208Pb、232Th、235U、238U,总的扫描时间约为0.23 s。锆石样品固定在环氧树脂靶上,抛光后在超纯水中超声清洗,分析前用分析级甲醇擦拭样品表面。采用5个激光脉冲对每个剥蚀区域进行预剥蚀(剥蚀深度约0.3 μm),以去除样品表面可能的污染。在束斑直径30 μm、剥蚀频率5 Hz、能量密度2 J/cm2的激光条件下分析样品。数据处理采用Iolite程序[36],锆石91500作为校正标样,GJ-1作为监测标样,每隔10~12个样品点分析2个91500标样及1个GJ-1标样。通常采集20 s的气体空白,35~40 s的信号区间进行数据处理,按指数方程进行深度分馏校正[36]。以玻璃标样NIST 610作为外标,91Zr作为内标计算微量元素含量。本次实验过程中测定的91500(1061.5±3.2 Ma, 2σ)、GJ-1 (604±6 Ma, 2σ)年龄在不确定范围内与推荐值一致。锆石年龄选择按照如下原则:对于<1000 Ma的年龄选取206Pb/238U计算值,对于>1000 Ma的年龄选取207Pb/206Pb计算值[37]。
3. 结果与讨论
3.1 碎屑锆石形态及U-Pb年龄
3.1.1 锆石形态
从研究样品锆石CL图像可以看出,碎屑锆石粒径变化较大,范围为20~200 μm。磨圆度从圆形到棱角均有分布,以次圆形和次棱角居多。研究样品中部分锆石具有清晰的岩浆岩振荡环带,如图3中C717-70、S256-112、S256-62、D275-111和S80-91,其中后两者可能为低温条件下微量元素的扩散速度慢而形成。样品中也有部分变质锆石,如无分带的D235-94和具有变质增生边的C717-52和D945-68两个样品(图3)。
3.1.2 碎屑锆石U-Pb年龄特征
锆石Th、U含量及Th/U值能够大致反映锆石的成因[38]。通常认为,岩浆锆石中Th、U含量较高且Th/U值较大,一般>0.3,而变质锆石的Th、U含量相对较低,一般<0.1。用于本文研究的85%以上的沉积物锆石Th/U>0.3,仅少数的Th/U<0.1(图4),说明大多数锆石为岩浆成因,能够反映其结晶年龄。
南黄海中部泥质区南部表层沉积物和SYS90-1A孔沉积物碎屑锆石U-Pb年龄谐和图如图5所示,各样品中年龄谐和锆石数量均超过90%,文中所用沉积物锆石年龄谐和度均>90%。9个沉积物样品锆石U-Pb年龄测试结果显示,根据年龄分布大致可分为两组:第一组表现出<100 Ma、100~300、300~500、600~1100、1800~2000和2300~2700 Ma等6个年龄区间,样品包括表层沉积物SYB86、SYB198、SYB256和钻孔沉积物SYS90-1A-C717、SYS90-1A-D235;第二组表现出<200、200~300、350~500、600~1100、1800~2000和2000~2600 Ma等6个主要年龄区间,样品包括表层沉积物SYB80和钻孔沉积物SYS90-1A-B709、SYS90-1A-D275、SYS90-1A-D945。各样品不同年龄区间锆石比例见表2和表3。
表 2 第一组样品不同年龄区间锆石比例Table 2. The proportion of zircons in different ages of the first group of samples% 样品编号 <100 Ma 100~300 Ma 300~500 Ma 600~1100 Ma 1300~1500 Ma 1800~2000 Ma 2300~2700 Ma SYS90-1A-C717 1 27 13 27 4 16 12 SYS90-1A-D235 4 26 11 35 2 6 17 SYB86 0 44 11 18 4 18 5 SYB198 3 54 13 21 2 5 2 SYB256 3 33 13 18 3 19 11 表 3 第二组样品不同年龄区间锆石比例Table 3. The proportion of zircons in different ages of the second group of samples% 样品编号 <200 Ma 200~300 Ma 350~500 Ma 600~1100 Ma 1300~1500 Ma 1800~2000 Ma 2000~2600 Ma SYS90-1A-B709 4 21 11 23 6 23 13 SYS90-1A-D275 3 13 12 41 3 16 13 SYS90-1A-D945 2 22 13 26 2 22 15 SYB80 2 21 9 35 4 15 15 3.2 沉积物碎屑锆石年龄物源判别
3.2.1 物源判别
南黄海周边的黄河、长江和朝鲜半岛的汉江、锦江、蟾津江等河流可能为海底沉积物的主要输送通道,本文以上述河流为端元进行物源判别。
首先,利用IsoplotR软件[39]绘制了研究样品与周边河流样品的KDE图(图6),结合各样品不同年龄区间的锆石比例,初步认为表层沉积物SYB86、SYB198、SYB256和钻孔沉积物SYS90-1A-C717、SYS90-1A-D235与长江输送沉积物的锆石U-Pb年龄谱比较相似,以200~300和700~1000 Ma为两组主要年龄,且多数样品含有<65 Ma年龄的锆石颗粒。其中年龄<65 Ma(新生代)的锆石样品占比不高,但明显说明这一组样品与其他样品的差异。长江上游流经的昌都地块和松潘-甘孜褶皱带及下游流经的下扬子板块均出露新生代岩体[40]。
表层沉积物SYB80和钻孔沉积物SYS90-1A-B709、SYS90-1A-D275、SYS90-1A-D945与黄河输送沉积物的锆石U-Pb年龄谱比较相似,以200~300、350~500、600~1100、1800~2000和2000~2600 Ma为主要的年龄峰,年龄峰的增加可能主要是受源区拓展的影响[43]。600~1100 Ma为长江输送沉积物的主要年龄峰。长江流域700~1000 Ma的锆石含量均较高,主要是有大量扬子克拉通和秦岭-大别造山带[44-45]碎屑沉积物输入所致。
研究样品与朝鲜半岛三条河流输送物质的锆石U-Pb年龄谱区别较大,应该不存在物源关系。朝鲜半岛河流锆石U-Pb年龄谱以100~250和1800~2000 Ma两组年龄峰为特征[33],与长江、黄河输送物质的锆石U-Pb年龄谱区别明显。
Vermeesch研究认为,基于多维定标法(MDS)的碎屑锆石U-Pb年龄相似性量化分析,能够实现年龄分布的有效捕捉,具有很强的实用性[46]。该基于Kolmogorov-Smirnoff(K-S)检验的D值或Kuiper检验的V值,通过特定的算法,将分析结果以点的形式投射在多维空间(二维或三维)中,表示多个样本之间的相对差异,从而显著提升碎屑锆石样品量化分析结果的可视化效果。样品间的差异性(δ)矩阵被函数f转换为一个由直线距离(d)表示的差异矩阵,对于两个样品i和j,其定义如下:
$$ d_{ij} {\text{≈}} f (\sigma_{ij}) $$ (1) 式(1)中:f (δij)是单调递增的转换函数,即i和j样品的差异性越大,多维空间中代表2个样品的点之间的距离也就越大。MDS利用这些差异矩阵将样品点投射在二维或三维空间中绘制成图。
利用IsoplotR软件绘制了研究样品与长江、黄河以及朝鲜半岛汉江、锦江、蟾津江等河流的MDS图(图7)。从图7中可以看出表层沉积物SYB80和钻孔沉积物SYS90-1A-B709、SYS90-1A-D275、SYS90-1A-D945与黄河沉积物的锆石U-Pb年龄比较靠近。表层沉积物SYB86、SYB198、SYB256和钻孔沉积物SYS90-1A-C717、SYS90-1A-D235与长江沉积物的锆石U-Pb年龄比较靠近。所有样品与朝鲜半岛河流相距均较远。韩国学者[33]对黄海表层沉积物锆石U-Pb年龄分析结果显示(图7b),南黄海南部大致以济州岛西缘为界,以西区域沉积物主要来自中国大陆,以东区域沉积物主要来自朝鲜半岛,这一界限大致与南黄海东侧粉砂区与砂质区分界重合。
![]() 图 7 物源分析图a. 研究样品与周边河流样品MDS图,b. 基于K-S检验南黄海南部表层样品物源分析[33],c. 样品位置图。Figure 7. Provenance analysisa:MDS plot of study samples and surrounding river samples; b:Possible provenance discrimination of southeastern Yellow Sea sandy sediments using the Kolmogorov-Smirnoff (K-S) test[33]; c:Sample location.
图 7 物源分析图a. 研究样品与周边河流样品MDS图,b. 基于K-S检验南黄海南部表层样品物源分析[33],c. 样品位置图。Figure 7. Provenance analysisa:MDS plot of study samples and surrounding river samples; b:Possible provenance discrimination of southeastern Yellow Sea sandy sediments using the Kolmogorov-Smirnoff (K-S) test[33]; c:Sample location.3.2.2 沉积动力机制对物源的控制
南黄海中部泥质区的形成受海平面变化、海洋环流、东亚季风、河流改道及河口三角洲形成等多种因素的影响[47-53]。其中,以黄海暖流和两侧沿岸流为主的南黄海环流体系,控制了周边河流输入物质的搬运和沉积[54]。北上的黄海暖流与南下的沿岸流相互作用形成逆时针旋转的气旋型涡旋,称为冷水团或冷涡,对南黄海中部泥质区的形成具有明显的控制作用[55]。诸多学者对黄海暖流的形成时间进行了研究[8,48,56-57],将其进入时间限定在6.9~4.3 kaBP。南黄海柱状沉积物环境磁学参数研究发现,黄海暖流进入以前南黄海沉积物可能主要由黄河供应,之后长江源物质的影响相对增加[58]。黄河入海物质向南搬运的沉积动力主要为冬季风驱动的沿岸流。黄海沿岸流携带的悬浮物质沉积形成南黄海中部泥质区,冬季风增强则加剧沉积物的再悬浮,使得悬浮体浓度增加、粒度变粗。长江入海物质沉积动力主要为夏季风和黄海暖流,二者共同作用于长江冲淡水[59],将其携带的物质向西北输运,沿岸流和黄海暖流共同影响南黄海沉积环境过程[60-61]。苏北-南黄海盆地和现代长江三角洲地层厚度的显著变化反映了河口的迁移,在低海平面时期长江分支河流直接向南黄海中部泥质区输送物质[62]。
通过南黄海中部泥质区表层沉积物碎屑锆石U-Pb年龄分析,发现中部泥质区为一个混合沉积区,在泥质区内部以黄河输送物质占主导,而在泥质区南侧,以长江输送物质居多。黄河和长江输送物质在南黄海中部泥质区的主控分界大致位于33.4°N附近。
3.2.3 晚第四纪沉积环境演化
CSDP-2孔位于南黄海中部隆起区,上部592.00 m 岩芯为松散沉积物,刘健等[9]对其开展了详细的古地磁、沉积相以及第四纪地质研究,可以作为南黄海第四纪地质研究的标准钻孔。钻孔研究结果显示,南黄海在新近纪的剥蚀止于约5.2 Ma,从约5.2 Ma至约1.7 Ma主要发育陆相地层。约1.7 Ma开始浙闽隆起逐渐沉降,至约0.83 Ma南黄海首次接受海侵,发育潮坪-滨岸相与河流相交互地层。约0.83 Ma开始,浙闽隆起沉降加剧,南黄海在间冰期高海平面时期的海洋环境基本接近现今环境。通过与周边QC2 孔[63]、SYS-0702 孔[64]和CSDP-1孔[65-66]等进行对比,发现南黄海西部陆架冷水团沉积在MIS5时期发育范围比现今更为广泛,之后则依次发育河流相、三角洲相、河流相和滨岸-陆架相沉积[9]。
本文利用碎屑锆石U-Pb定年开展物源分析显示,钻孔沉积物可以分为3段:第一段从钻孔底部至69.68 m,沉积物可能来自黄河输送入海物质;第二段从56.20 m到68.88 m,沉积物碎屑锆石U-Pb年龄特征与长江源物质相似度较高,说明该时段长江源物质对研究区贡献较大;第三段从56.20 m至钻孔顶部,沉积物主要为黄河源物质。据古地磁推测,SYS90-1A孔底界年龄约为1.0 Ma。利用天文年代调谐确定56.20 ~68.88 m沉积物具体时代为 0.59~0.71 Ma。
CSDP-1孔的研究结果显示,0.83 Ma开始南黄海发生大规模区域性海侵,南黄海海洋环境与现今接近,在此之前南黄海区域沉积物以长江物质为主[62]。南黄海NHH01孔的研究结果表明[15],0.88 Ma以来才开始出现黄河源物质信号,这与本文的研究结果较为一致,说明黄河源物质大致在0.88~1.0 Ma开始影响南黄海。对照全球海平面变化曲线(图2),0.59~0.71 Ma为低海平面时期,SYS90-1A孔沉积物在该时段主要受长江控制,推测与海平面下降及长江入海口向海推进有关。综合CSDP-1孔、NHH01孔和SYS90-1A孔(钻孔位置见图1)沉积环境对比及物源分析结果,在南黄海地区,受浙闽隆起沉降影响,约1.0~0.83 Ma期间,海水从东南方向以“通道”式进入南黄海,不同区域存在“同期异象”现象。至晚更新世,受全球海平面变化影响,3个钻孔沉积环境变化一致。
4. 结论
南黄海中部泥质区南部锆石U-Pb年龄特征指示沉积物主要由黄河供应,而泥质区以南区域长江源物质占主导。早更新世晚期以来沉积物不同时期物源差异明显,其中,中更新世早期(0.59~0.71 Ma)以长江源物质为主,早更新晚期至中更新世以及中更新世中期以来以黄河源物质为主。上述表层沉积物样品和钻孔沉积物样品碎屑锆石U-Pb年龄分布与朝鲜半岛河流沉积物完全不同。
早期关于南黄海早更新世以来沉积物物源的认识主要以黄河源物质为主,长江源物质对南黄海泥质区沉积物的影响范围不够清晰。本文通过地质浅钻揭示出长江源物质在早更新世晚期和中更新世早期(0.59~0.71 Ma)对南黄海泥质区贡献较大,但难以从区域上识别出这一阶段长江源物质的影响范围。且受取样密度所限,可能对SYS90-1A孔中长江源物质占主导时期的揭示有所遗漏,后期需要开展进一步研究。
致谢:感谢自然资源部国际合作司提供支持。
-
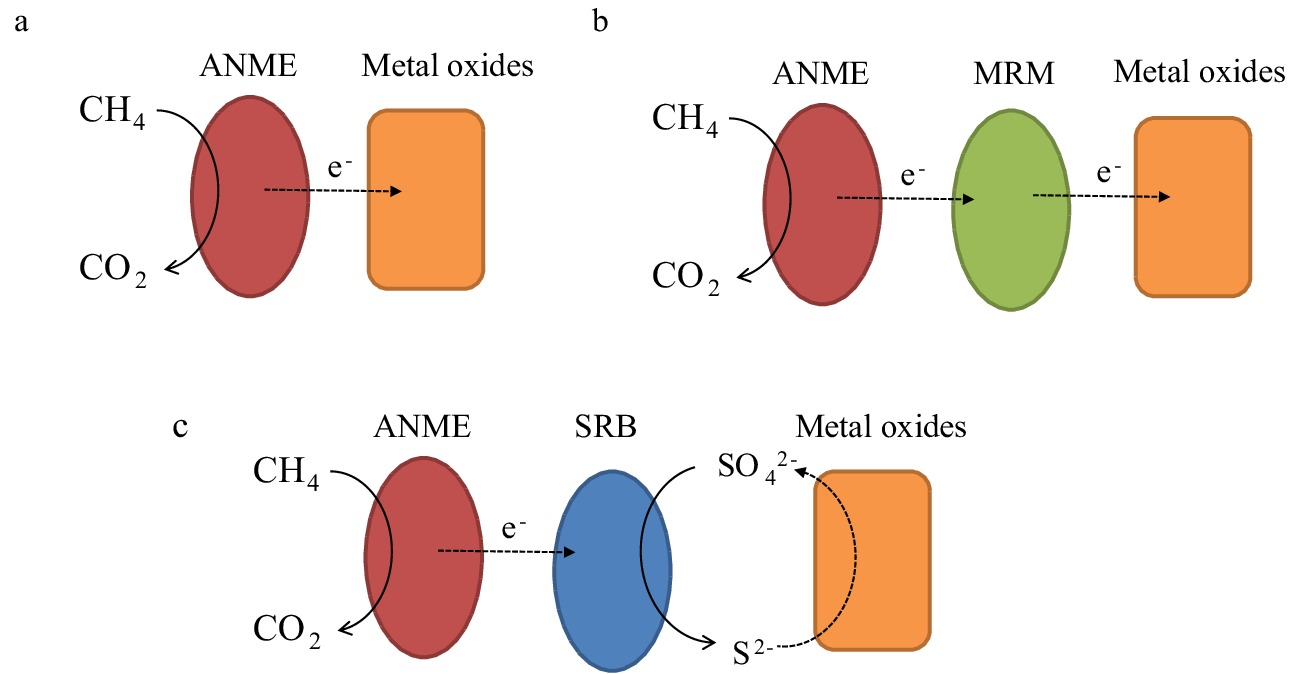
图 6 微生物介导Metal-AOM的不同反应机制[76]
a. ANME单独负责Metal-AOM, b. ANME与合作伙伴MRM的合作进行Metal-AOM, c. 金属氧化物促进S-DAOM的Metal-AOM。
Figure 6. Different mechanisms of microbe-mediated metal-AOM
a. metal-AOM by ANME alone, b. metal-AOM by cooperation between ANME and partner MRM, c. apparent metal-AOM by the stimulation of metal oxides on sulfate-AOM .
表 1 不同电子受体类型甲烷氧化反应的吉布斯自由能[45-46]
Table 1 Standard Gibbs free energies with different electron acceptors for methane oxidation [45-46]
不同电子受体介导的甲烷氧化反应 吉布斯自由能/(kJ·mol−1 CH4) CH4+2O2→CO2+2H2O −858.7 CH4+SO42−→HCO3−+HS−+H2O −33.0 CH4+4NO3−→HCO3−+4NO2−+H++H2O −483.4 3CH4+8NO2−+8H+→ 4N2+3CO2+10H2O −928.0 CH4+8Fe3++2H2O→CO2+8Fe2++8H+ −471.0 5CH4+8MnO4−+19H+→5HCO3-+8Mn2++17H2O −1 008.1 -
[1] IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change[M]. Cambridge, UK: Cambridge University Press, 2004.
[2] Rice D D. Biogenic gas: controls, habitats, and resource potential[M]//Howell D G. The Future of Energy Gases. Washington: United States Government Printing Office, 1993: 583-606.
[3] Hinrichs K U, Boetius A. The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry[M]//Wefer G, Billett D, Hebbeln D, et al. Ocean Margin Systems. Berlin: Springer, 2002: 457-477.
[4] Reeburgh W S. Oceanic methane biogeochemistry [J]. Chemical Reviews, 2007, 107(2): 486-513. doi: 10.1021/cr050362v
[5] Reeburgh W S. Methane consumption in Cariaco Trench waters and sediments [J]. Earth and Planetary Science Letters, 1976, 28(3): 337-344. doi: 10.1016/0012-821X(76)90195-3
[6] Islas-Lima S, Thalasso F, Gómez-Hernandez J. Evidence of anoxic methane oxidation coupled to denitrification [J]. Water Research, 2004, 38(1): 13-16. doi: 10.1016/j.watres.2003.08.024
[7] Beal E J, House C H, Orphan V J. Manganese- and iron-dependent marine methane oxidation [J]. Science, 2009, 325(5937): 184-187. doi: 10.1126/science.1169984
[8] Kallmeyer J, Pockalny R, Adhikari R R, et al. Global distribution of microbial abundance and biomass in subseafloor sediment [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(40): 16213-16216. doi: 10.1073/pnas.1203849109
[9] Liu Y C, Whitman W B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea [J]. Annals of the New York Academy of Sciences, 2008, 1125(1): 171-189. doi: 10.1196/annals.1419.019
[10] Adam P S, Borrel G, Brochier-Armanet C, et al. The growing tree of Archaea: new perspectives on their diversity, evolution and ecology [J]. The ISME Journal, 2017, 11(11): 2407-2425. doi: 10.1038/ismej.2017.122
[11] Bapteste É, Brochier C, Boucher Y. Higher-level classification of the Archaea: evolution of methanogenesis and methanogens [J]. Archaea, 2005, 2005: 859728.
[12] Borrel G, Parisot N, Harris H M, et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine [J]. BMC Genomics, 2014, 15: 679. doi: 10.1186/1471-2164-15-679
[13] Nobu M K, Narihiro T, Kuroda K, et al. Chasing the elusive Euryarchaeota class WSA2: genomes reveal a uniquely fastidious methyl-reducing methanogen [J]. The ISME Journal, 2016, 10(10): 2478-2487. doi: 10.1038/ismej.2016.33
[14] Sorokin D Y, Makarova K S, Abbas B, et al. Discovery of extremely halophilic, methyl-reducing euryarchaea provides insights into the evolutionary origin of methanogenesis [J]. Nature Microbiology, 2017, 2(8): 17081. doi: 10.1038/nmicrobiol.2017.81
[15] Evans P N, Parks D H, Chadwick G L, et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics [J]. Science, 2015, 350(6259): 434-438. doi: 10.1126/science.aac7745
[16] Vanwonterghem I, Evans P N, Parks D H, et al. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota [J]. Nature Microbiology, 2016, 1: 16170. doi: 10.1038/nmicrobiol.2016.170
[17] Wang Y L, Hua Z S, Goh K M, et al. Further expansion of methane metabolism in the Archaea. BioRxiv, 2018.
[18] Conklin A, Stensel H D, Ferguson J. Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion [J]. Water Environment Research, 2006, 78(5): 486-496. doi: 10.2175/106143006X95393
[19] Kobayashi T, Yasuda D, Li Y Y, et al. Characterization of start-up performance and archaeal community shifts during anaerobic self-degradation of waste-activated sludge [J]. Bioresource Technology, 2009, 100(21): 4981-4988. doi: 10.1016/j.biortech.2009.05.043
[20] 段昌海, 张翠景, 孙艺华, 等. 新型产甲烷古菌研究进展[J]. 微生物学报, 2019, 59(6):981-995 DUAN Changhai, ZHANG Cuijing, SUN Yihua, et al. Recent advances on the novel methanogens [J]. Acta Microbiologica Sinica, 2019, 59(6): 981-995.
[21] Zhou Z, Zhang C J, Liu P F, et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species [J]. Nature, 2022, 601(7892): 257-262. doi: 10.1038/s41586-021-04235-2
[22] Konhauser K O. Introduction to Geomicrobiology[M]. John Wiley & Sons, 2009.
[23] Xiao K Q, Beulig F, Kjeldsen K U, et al. Concurrent methane production and oxidation in surface sediment from Aarhus Bay, Denmark [J]. Frontiers in Microbiology, 2017, 8: 1198. doi: 10.3389/fmicb.2017.01198
[24] Xiao K Q, Beulig F, Røy H, et al. Methylotrophic methanogenesis fuels cryptic methane cycling in marine surface sediment [J]. Limnology and Oceanography, 2018, 63(4): 1519-1527. doi: 10.1002/lno.10788
[25] Zhuang G C, Heuer V B, Lazar C S, et al. Relative importance of methylotrophic methanogenesis in sediments of the Western Mediterranean Sea [J]. Geochimica et Cosmochimica Acta, 2018, 224: 171-186. doi: 10.1016/j.gca.2017.12.024
[26] Oremland R S, Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments [J]. Applied and Environmental Microbiology, 1982, 44(6): 1270-1276. doi: 10.1128/aem.44.6.1270-1276.1982
[27] Li L Y, Zhang W T, Zhang S J, et al. Bacteria and archaea synergistically convert glycine betaine to biogenic methane in the Formosa cold seep of the South China sea [J]. Msystems, 2021, 6(5): e0070321. doi: 10.1128/mSystems.00703-21
[28] Dolfing J, Larter S R, Head I M. Thermodynamic constraints on methanogenic crude oil biodegradation [J]. The ISME Journal, 2008, 2(4): 442-452. doi: 10.1038/ismej.2007.111
[29] Ozuolmez D, Na H, Lever M A, et al. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: teamwork or coexistence? [J]. Frontiers in Microbiology, 2015, 6: 492.
[30] Chen Y, Wu N Y, Liu C L, et al. Methanogenesis pathways of methanogens and their responses to substrates and temperature in sediments from the South Yellow Sea [J]. Science of the Total Environment, 2022, 815: 152645. doi: 10.1016/j.scitotenv.2021.152645
[31] Hanson R S, Hanson T E. Methanotrophic bacteria [J]. Microbiological Reviews, 1996, 60(2): 439-471. doi: 10.1128/mr.60.2.439-471.1996
[32] Dedysh S N, Knief C, Dunfield P F. Methylocella species are facultatively methanotrophic [J]. Journal of Bacteriology, 2005, 187(13): 4665-4670. doi: 10.1128/JB.187.13.4665-4670.2005
[33] Vorobev A V, Baani M, Doronina N V, et al. Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase [J]. International Journal of Systematic and Evolutionary Microbiology, 2011, 61(10): 2456-2463. doi: 10.1099/ijs.0.028118-0
[34] Elsaied H E, Hayashi T, Naganuma T. Molecular analysis of deep-sea hydrothermal vent aerobic methanotrophs by targeting genes of 16S rRNA and particulate methane monooxygenase [J]. Marine Biotechnology, 2004, 6(5): 503-509. doi: 10.1007/s10126-004-3042-0
[35] Tavormina P L, Ussler III W, Orphan V J. Planktonic and sediment-associated aerobic methanotrophs in two seep systems along the North American margin [J]. Applied and Environmental Microbiology, 2008, 74(13): 3985-3995. doi: 10.1128/AEM.00069-08
[36] Wasmund K, Kurtböke D I, Burns K A, et al. Microbial diversity in sediments associated with a shallow methane seep in the tropical Timor Sea of Australia reveals a novel aerobic methanotroph diversity [J]. FEMS Microbiology Ecology, 2009, 68(2): 142-151. doi: 10.1111/j.1574-6941.2009.00667.x
[37] Alperin M J, Hoehler T M. Anaerobic methane oxidation by archaea/sulfate-reducing bacteria aggregates: 1. Thermodynamic and physical constraints [J]. American Journal of Science, 2009, 309(10): 869-957. doi: 10.2475/10.2009.01
[38] Knittel K, Lösekann T, Boetius A, et al. Diversity and distribution of methanotrophic archaea at cold seeps [J]. Applied and Environmental Microbiology, 2005, 71(1): 467-479. doi: 10.1128/AEM.71.1.467-479.2005
[39] Haroon M F, Hu S H, Shi Y, et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage [J]. Nature, 2013, 500(7464): 567-570. doi: 10.1038/nature12375
[40] Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process [J]. Annual Review of Microbiology, 2009, 63: 311-334. doi: 10.1146/annurev.micro.61.080706.093130
[41] Timmers P H A, Widjaja-Greefkes H C A, Plugge C M, et al. Evaluation and optimization of PCR primers for selective and quantitative detection of marine ANME subclusters involved in sulfate-dependent anaerobic methane oxidation [J]. Applied Microbiology and Biotechnology, 2017, 101(14): 5847-5859. doi: 10.1007/s00253-017-8338-x
[42] Kong Y, Lei H Y, Zhang Z L, et al. Depth profiles of geochemical features, geochemical activities and biodiversity of microbial communities in marine sediments from the Shenhu area, the northern South China Sea [J]. Science of the Total Environment, 2021, 779: 146233. doi: 10.1016/j.scitotenv.2021.146233
[43] Yang S S, Lv Y X, Liu X P, et al. Genomic and enzymatic evidence of acetogenesis by anaerobic methanotrophic archaea [J]. Nature Communications, 2020, 11(1): 3941. doi: 10.1038/s41467-020-17860-8
[44] Metcalfe K S, Murali R, Mullin S W, et al. Experimentally-validated correlation analysis reveals new anaerobic methane oxidation partnerships with consortium-level heterogeneity in diazotrophy [J]. The ISME Journal, 2021, 15(2): 377-396. doi: 10.1038/s41396-020-00757-1
[45] Caldwell S L, Laidler J R, Brewer E A, et al. Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms [J]. Environmental Science & Technology, 2008, 42(18): 6791-6799.
[46] Ettwig K F, Butler M K, Le Paslier D, et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria [J]. Nature, 2010, 464(7288): 543-548. doi: 10.1038/nature08883
[47] Roalkvam I, Jørgensen S L, Chen Y F, et al. New insight into stratification of anaerobic methanotrophs in cold seep sediments [J]. FEMS Microbiology Ecology, 2011, 78(2): 233-243. doi: 10.1111/j.1574-6941.2011.01153.x
[48] Yanagawa K, Sunamura M, Lever M A, et al. Niche separation of methanotrophic archaea (ANME-1 and -2) in methane-seep sediments of the eastern Japan Sea offshore Joetsu [J]. Geomicrobiology Journal, 2011, 28(2): 118-129. doi: 10.1080/01490451003709334
[49] Chen Y, Xu C L, Wu N Y, et al. Diversity of anaerobic methane oxidizers in the cold seep sediments of the Okinawa Trough [J]. Frontiers in Microbiology, 2022, 13: 819187. doi: 10.3389/fmicb.2022.819187
[50] Niu M Y, Fan X B, Zhuang G C, et al. Methane-metabolizing microbial communities in sediments of the Haima cold seep area, northwest slope of the South China Sea [J]. FEMS Microbiology Ecology, 2017, 93(9): fix101.
[51] Lv Y X, Yang S S, Xiao X, et al. Stimulated organic carbon cycling and microbial community shift driven by a simulated cold-seep eruption [J]. mBio, 2022, 13(2): e0008722. doi: 10.1128/mbio.00087-22
[52] Hoehler T M, Alperin M J, Albert D B, et al. Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium [J]. Global Biogeochemical Cycles, 1994, 8(4): 451-463. doi: 10.1029/94GB01800
[53] Hallam S J, Putnam N, Preston C M, et al. Reverse methanogenesis: testing the hypothesis with environmental genomics [J]. Science, 2004, 305(5689): 1457-1462. doi: 10.1126/science.1100025
[54] 陈颖. 厌氧甲烷氧化微生物代谢分子机制及其潜在参与矿物形成机理的研究[D]. 上海交通大学博士学位论文, 2014. CHEN Ying. Molecular metabolism study on microbial anaerobic methane oxidation and the associated biogenic minerals[D]. Doctor Dissertation of Shanghai Jiao Tong University, 2014.
[55] Valentine D L, Reeburgh W S. New perspectives on anaerobic methane oxidation: minireview [J]. Environmental Microbiology, 2000, 2(5): 477-484. doi: 10.1046/j.1462-2920.2000.00135.x
[56] Moran J J, Beal E J, Vrentas J M, et al. Methyl sulfides as intermediates in the anaerobic oxidation of methane [J]. Environmental Microbiology, 2008, 10(1): 162-173.
[57] Raghoebarsing A A, Pol A, van de Pas-Schoonen K T, et al. A microbial consortium couples anaerobic methane oxidation to denitrification [J]. Nature, 2006, 440(7086): 918-921. doi: 10.1038/nature04617
[58] Ettwig K F, Shima S, van de Pas-Schoonen K T, et al. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea [J]. Environmental Microbiology, 2008, 10(11): 3164-3173. doi: 10.1111/j.1462-2920.2008.01724.x
[59] Chen J, Jiang X W, Gu J D. Existence of novel phylotypes of nitrite-dependent anaerobic methane-oxidizing bacteria in surface and subsurface sediments of the South China Sea [J]. Geomicrobiology Journal, 2015, 32(1): 1-10. doi: 10.1080/01490451.2014.917742
[60] Padilla C C, Bristow L A, Sarode N, et al. NC10 bacteria in marine oxygen minimum zones [J]. The ISME Journal, 2016, 10(8): 2067-2071. doi: 10.1038/ismej.2015.262
[61] 吴忆宁, 梅娟, 沈耀良. 甲烷厌氧氧化机理及其应用研究进展[J]. 生态科学, 2018, 37(4):231-240 WU Yining, MEI Juan, SHEN Yaoliang. Research progress on microbial mechanism and application of anaerobic oxidation of methane [J]. Ecological Science, 2018, 37(4): 231-240.
[62] Hansen L B, Finster K, Fossing H, et al. Anaerobic methane oxidation in sulfate depleted sediments: effects of sulfate and molybdate additions [J]. Aquatic Microbial Ecology, 1998, 14(2): 195-204.
[63] Joye S B, Boetius A, Orcutt B N, et al. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps [J]. Chemical Geology, 2004, 205(3-4): 219-238. doi: 10.1016/j.chemgeo.2003.12.019
[64] Niemann H, Duarte J, Hensen C, et al. Microbial methane turnover at mud volcanoes of the Gulf of Cadiz [J]. Geochimica et Cosmochimica Acta, 2006, 70(21): 5336-5355. doi: 10.1016/j.gca.2006.08.010
[65] Parkes R J, Cragg B A, Banning N, et al. Biogeochemistry and biodiversity of methane cycling in subsurface marine sediments (Skagerrak, Denmark) [J]. Environmental Microbiology, 2007, 9(5): 1146-1161. doi: 10.1111/j.1462-2920.2006.01237.x
[66] Maignien L, Parkes R J, Cragg B, et al. Anaerobic oxidation of methane in hypersaline cold seep sediments [J]. FEMS Microbiology Ecology, 2013, 83(1): 214-231. doi: 10.1111/j.1574-6941.2012.01466.x
[67] Segarra K E A, Comerford C, Slaughter J, et al. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments [J]. Geochimica et Cosmochimica Acta, 2013, 115: 15-30. doi: 10.1016/j.gca.2013.03.029
[68] Ettwig K F, Zhu B L, Speth D, et al. Archaea catalyze iron-dependent anaerobic oxidation of methane [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(45): 12792-12796. doi: 10.1073/pnas.1609534113
[69] Scheller S, Yu H, Chadwick G L, et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction [J]. Science, 2016, 351(6274): 703-707. doi: 10.1126/science.aad7154
[70] Bar-Or I, Elvert M, Eckert W, et al. Iron-coupled anaerobic oxidation of methane performed by a mixed bacterial-archaeal community based on poorly reactive minerals [J]. Environmental Science & Technology, 2017, 51(21): 12293-12301.
[71] Cai C, Leu A O, Xie G J, et al. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction [J]. The ISME Journal, 2018, 12(8): 1929-1939. doi: 10.1038/s41396-018-0109-x
[72] Yan Z, Joshi P, Gorski C A, et al. A biochemical framework for anaerobic oxidation of methane driven by Fe(III)-dependent respiration [J]. Nature Communications, 2018, 9(1): 1642. doi: 10.1038/s41467-018-04097-9
[73] He Q X, Yu L P, Li J B, et al. Electron shuttles enhance anaerobic oxidation of methane coupled to iron(III) reduction [J]. Science of the Total Environment, 2019, 688: 664-672. doi: 10.1016/j.scitotenv.2019.06.299
[74] Liang L W, Wang Y Z, Sivan O, et al. Metal-dependent anaerobic methane oxidation in marine sediment: insights from marine settings and other systems [J]. Science China Life Sciences, 2019, 62(10): 1287-1295. doi: 10.1007/s11427-018-9554-5
[75] He Z F, Zhang Q Y, Feng Y D, et al. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane [J]. Science of the Total Environment, 2018, 610-611: 759-768. doi: 10.1016/j.scitotenv.2017.08.140
[76] Fu L, Li S W, Ding Z W, et al. Iron reduction in the DAMO/Shewanella oneidensis MR-1 coculture system and the fate of Fe(II) [J]. Water Research, 2016, 88: 808-815. doi: 10.1016/j.watres.2015.11.011
[77] Sivan O, Antler G, Turchyn A V, et al. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(40): E4139-E4147.
[78] Yang H L, Yu S, Lu H L. Iron-coupled anaerobic oxidation of methane in marine sediments: a review [J]. Journal of Marine Science and Engineering, 2021, 9(8): 875. doi: 10.3390/jmse9080875
-
期刊类型引用(1)
1. 马瑞琦,曹运诚,何雯,郑子涵,朱志伟,陈多福. 南海北部东沙海域GMGS2-16站位25 ka以来水合物稳定带和流体超压变化. 海洋地质前沿. 2025(01): 21-30 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: