Geochemical records of hydrothermal fluids in corals: Evidence of rare earth elements from coral reefs in the Yongxing Island, Xisha, South China Sea
-
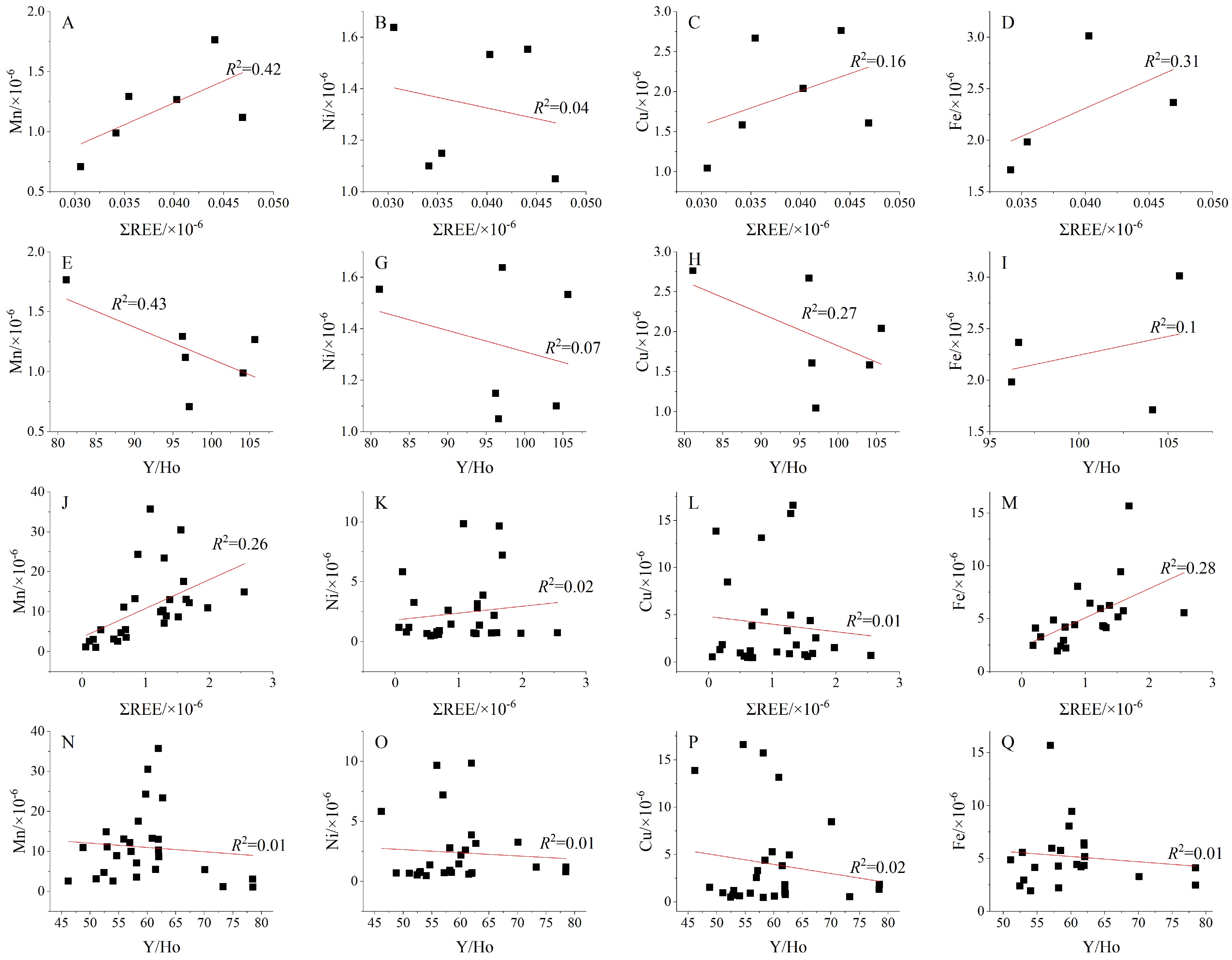
摘要: 碳酸盐岩中稀土元素的含量、配分模式及元素异常记录了周围沉积水体的特征,能够很好地指示古海洋及沉积环境。珊瑚具有的高分辨率和稀土元素的高稳定性的特点,能够忠实地记录周围海水的地球化学特征。本文以南海西沙宣德环礁永兴岛142~84 ka发育的珊瑚礁为研究对象,通过主微量元素含量,尤其是稀土元素含量及其配分图解,判断珊瑚礁形成时周围水体的特征。结果表明自142 ka以来,永兴岛大部分珊瑚礁具有正常海相碳酸盐岩的稀土配分特征,表现为LREE亏损,Ce负异常及高的Y/Ho比值,表明周围水体属于开阔的浅海,但是位于23 m处(年龄为114 ka)的滨珊瑚骨骼格架除了有正常海相碳酸盐岩的特征外,还具有明显的Eu正异常,这表明其形成时有热液流体的加入。经过模型计算,认为在滨珊瑚骨骼格架的生长阶段,至少有0.1%的热液加入周围的海水中。通过资料查询和年龄对比,认为这些热液可能与高尖石岛或海南岛火山活动有关。Abstract: The contents, distribution pattern and elemental anomalies of rare earth elements in carbonates are the records of surrounding water. Corals are characterized by high resolution and high stability of rare earth elements and may faithfully record the geochemical characteristics of the surrounding seawater. In this paper, we analyzed the coral reefs from 142 to 84 ka collected from the Yongxing Island of the Xuande Atoll of Xisha Islands, South China Sea. Trace element contents, especially the rare earth element contents and their distribution patterns are used in this paper to determine the characteristics of the sea water, in which the coral reefs grew. Results show that, since 142 ka, most of the coral reefs in the Yongxing Island has a normal rare earth element distribution pattern of marine carbonates, characterized by LREE depletion, negative Ce anomalies and high Y/Ho ratios, indicating an environment of open shallow sea. In contrast, the coral skeletons in depth of 23 m 114 ka have similar LREE depletion, negative Ce anomalies and high Y/Ho ratios, but positive Eu anomalies. This suggests that certain amount of hydrothermal fluid has been input during the growth of corals. Based on the model calculations, it is inferred that at least 0.1% of hydrothermal fluid has been added to the open seawater during that time. The hydrothermal fluids may be related to the volcanic activities observed at Gaojianshi island or Hainan island.
-
Keywords:
- coral /
- rare earth element /
- Eu anomaly /
- hydrothermal solution /
- South China Sea
-
珠江口盆地西江凹陷南部西江36洼与番禺4洼地理位置相邻(图1a),勘探结果却截然不同,番禺4洼是一个典型的“小而肥”富生烃洼陷,探明储量已超过亿吨[1-3]。相比而言,西江36洼截止目前尚未有商业发现,油气勘探前景非常不明朗。
目前针对番禺4洼文昌组层序地层及沉积体系已有多期研究[1,3-5],对于西江36洼文昌组研究较少,未见相关报道。该地区最新重采集处理的三维地震资料已覆盖西江36洼与番禺4洼整个地区,区域地层研究表明两个洼陷在文昌组沉积时期形成了统一的洼陷,因而笔者尝试将西江36洼与番禺4洼作为一个整体开展文昌组层序地层及沉积体系研究,通过对比两个洼陷文昌期各三级层序的烃源条件,来预测西江36洼烃源潜力。
本次研究在沉积学、层序地层学及地震沉积学理论的指导下,充分利用西江36洼与番禺4洼钻遇文昌组的井岩心、测录井、分析化验资料以及覆盖全区三维地震资料,建立研究区文昌组三级层序地层格架,确定各三级层序主要相带类型及展布特征,在此基础上对比两个洼陷不同时期半深湖—深湖相规模及物源输入量发育情况,推测西江36洼文昌组烃源潜力,以期为下一步勘探部署提供依据。
1. 区域地质背景
珠江口盆地处于南海北部陆缘中部,是中生代末期以来在伸展断陷基础上发育的被动大陆边缘盆地,呈NE-NEE向展布。盆地经历了3个构造演化阶段:古新世—始新世的裂陷阶段、渐新世—中中新世的拗陷阶段和中中新世以后的块断升降阶段[6],自下而上依次发育古新统(神狐组)、中始新统(文昌组)、上始新统(恩平组)、渐新统(珠海组)、中新统(珠江组)、中中新统(韩江组)、上中新统(粤海组)和上新统(万山组)。盆地由“三隆三坳”组成,自北向南包括北部隆起带、北部坳陷带、中央隆起带、中部坳陷带、南部隆起带和南部坳陷带,在平面上形成了“南北分带、东西分块”的构造格局[7-10]。珠一坳陷位于珠江口盆地北部坳陷带,走向为NE向,大致与海岸线平行,其内发育的北西向低凸起及北东向断裂体系共同控制了内部具有东西分块构造特征的凹陷分布格局。凹陷分布自西向东依次为恩平凹陷、西江凹陷、惠州凹陷、陆丰凹陷和韩江凹陷(图1a)。
本次研究的西江36洼与番禺4洼位于珠一坳陷西江凹陷南部,西靠恩西低凸起,东侧以惠西低凸起分界,南接番禺低凸起及东沙隆起,北侧与西江主洼相邻,整体是个东南断西北超、呈北东(或北东东)走向复式箕状洼陷,研究区内正断层广泛分布,以北东(或北东东)向边界主干断裂为主,同时后期发育的北西(或北西西)向断裂体系共同控制了其隆洼相间的构造格局。由于盆地发育时期一直受构造活动影响强烈,文昌组内部也经历多次构造及火山活动,遭受过地层出露剥蚀。西江36洼与番禺4洼研究区主要包括西江36东次洼、西江36西次洼、番禺4北次洼、番禺4南次洼、番禺4西次洼、番禺4西北次洼等次级构造单元(图1b)。研究区目的层为文昌组,由于该区域不发育神狐组,文昌组沉积在前古近系基底之上。
2. 层序地层格架及充填特征
2.1 层序地层划分方案
笔者在综合研究地震、岩心及测录井等资料的基础上,通过井震标定,结合前人研究成果,对西江36洼与番禺4洼开展了层序地层划分对比。研究表明文昌组整体是一个二级层序,进一步可分为两个准二级层序(上文昌、下文昌),及划分出6个三级层序,自下而上依次为文六段至文一段,对应界面自下而上依次为Tg至T80(图2),其中底、顶界面Tg、T80为二级层序界面,分别响应于珠琼运动一幕、珠琼运动二幕构造事件;T83为准二级层序界面,响应于惠州运动[11],其他为三级层序界面。文昌组对应裂陷Ⅰ幕沉积[6],包含裂陷初始期、裂陷扩展期、裂陷强烈期、裂陷转换期、裂陷收缩期、裂陷萎缩期的沉积充填演化过程[12],同时又由T83界面分为下文昌、上文昌两个准二级层序,分别对应裂陷ⅠA幕和裂陷ⅠB幕。其中西江36洼发育文六段、文五段、文四段、文三段、文二段,番禺4洼发育文五段、文四段、文三段、文二段、文一段,层序格架方案见图2。
2.2 层序界面的识别
2.2.1 层序界面的地震反射特征
(1)构造层序界面
西江36洼和番禺4洼文昌组底、顶界面为二级层序界面,对应于地震反射界面Tg、T80。Tg为基底和古近系分界面,由珠琼一幕构造运动产生的不整合界面。界面上下地层反射特征及断裂发育特征存在明显的差异。Tg界面之下主要为中生界花岗岩杂乱空白反射;Tg界面之上主要表现为水平或楔状充填反射,普遍可见上超或远端上超反射,为典型的文昌组裂陷期沉积特征,与基底以下地层有着显著差异(图3)。
T80界面是文昌组顶界面,为珠琼二幕构造运动产生的不整合界面。T80界面主要表现为典型的“上超下削”特征,地震上表现为中—强振幅高连续反射,表现为两种界面特征,削截不整合面、上超不整合面,在洼陷内可见明显的削截或不整合。区域削截特征主要分布在番禺4北次洼、番禺4南次洼、番禺4西次洼,上超分布在番禺4南次洼、番禺4西次洼、番禺4西北次洼、西江36西次洼、西江36东次洼等地区。此外,部分区域T80界面上下表现为明显的地震振幅差异(图3)。
T83为上、下文昌组转换面,对应于文四段顶界面,是文昌组内部准二级层序界面,为惠州运动产生的不整合面[11]。由于该时期构造动力的转变,导致断裂活动、沉降作用等发生变化,进而引起湖盆水体变化、沉积充填转变和层序迁移,该界面在研究区各次洼可识别追踪。地震剖面上该界面表现为较高连续、强振幅反射,界面之上可见上超,受隆起影响,洼陷边缘之下可见削截反射,界面上下地震相差异明显,地层倾角也发生改变,同时界面上下沉积中心从东北向西南方向迁移(图3-4)。
(2)三级层序界面
西江36洼和番禺4洼文昌组三级层序界面包括T85、T84、T82、T81等4个界面,主要由湖平面变化以及与之伴生的沉积物供应速率变化造成的,三级层序界面在井震结合基础上依据上超反射终止方式、地震相及产状差异识别[13-17](图3)。
T85对应于文六段顶界面,该界面从陡坡带向缓坡带上超反射,仅发育在西江36洼,T84界面对应于文五段顶界面,界面之上可见从陡坡带向缓坡带上超反射,界面上下地震相特征差异明显,界面之下为弱振幅,界面之上见一套强振幅地质体;T82界面相当于文三段顶界面,形成于弱断陷时期, 地震相表现为中—强振幅、中—高连续不整合面,主要界面之上可见上超现象。T81不整合面相当于文二段顶界面,该时期洼陷断层活动基本停止,洼陷整体填平补齐。地震界面为中—高连续、中—低振幅地震反射,界面之上可见上超,分布局限,仅在南次洼、西次洼、西北次洼孤立存在。
2.2.2 层序界面的钻井反射特征
研究区内有5口探井钻遇到文昌组,其中4口井钻遇Tg界面,未有井钻遇T85界面,1口井钻遇T84界面,4口井钻遇T83界面,3口井钻遇T82界面,1口井钻遇T81界面,5口井钻遇T80界面,Tg界面为火成岩及沉积岩的分界面。T80界面上下岩性差异明显,为泥岩、砂岩分界面,GR、DT界面上下基值变化明显,T80之上测井曲线为箱型、之下为齿化线型;同时古生物分析化验表明,T80之下可见代表湖相的浮游藻类,以及丰富的反映优质烃源无定型有机质。在测井曲线上,为正、反旋回转换面,位于一套砂岩顶部、泥岩底部,反映了基准面短暂下降到大幅升高的沉积过程。T84界面,测井曲线上为正反旋回分界面,界面之下多为粉砂质泥岩、粉砂岩、泥岩等较细岩性组成的反旋回,向上呈现岩性变细的正旋回,反映水体开始加深;T82界面测井曲线为正反旋回分界面,之下为泥岩、粉砂质泥岩、粉砂岩等较细岩性组成的反旋回,之上为细砂岩、泥岩等岩性向上颗粒变粗正旋回。T81界面测井曲线为反旋回顶部,界面上下岩性变化不大,皆为泥岩(图3)。
2.3 层序充填特征
番禺4洼—西江36洼文昌期处于断陷湖盆发育时期,其各三级层序沉积体系均以湖相沉积为特征,但各层序的充填序列特征受湖盆演化的阶段性控制。番禺4 洼文昌期发育时期一直受构造活动影响强烈,文昌组内部遭受多期次的地层出露剥蚀,致使现今存在的各层序基本为残留地层,缓坡带地层剥蚀较深洼带强烈。西江36洼整体上为继承性发育,火山活动不明显。通过构造控边断裂、洼陷结构特征及典型层序界面识别综合分析,认为番禺4洼与西江36洼之间存在一定的先后发育次序。其中,西江36洼发育文昌期第一个三级层序文六段,其界面特征相对清晰,沉积范围主要局限于控边断裂周缘,沉积范围较小,这个时期番禺4洼还未开始充填;从文五段至文二段,西江36洼及番禺4洼皆有发育,不同时期厚度有差异,在文五段至文四段,西江36洼沉积更厚,文三段至文二段,番禺4洼整体沉积更厚(图4);同时西江36洼先消失,不发育文一段,这个时期番禺4洼仍有沉积充填,因此,西江36洼与番禺4洼存在一定的构造-沉积迁移(图5)。
同时番禺4洼的西次洼及西北次洼的层序发育特征也有着相似规律。西次洼主要发育文五段至文一段共5个三级层序,而西北次洼则主要发育文四段至文一段等4个三级层序,但从其三级层序发育的厚度来看,同样存在从西次洼向西北次洼迁移现象(图4)。
3. 沉积相类型和沉积体系展布
3.1 沉积相类型
西江36洼和番禺4洼文昌组处于湖盆断陷期,通过对岩心、测录井及地震等资料的分析,研究区共识别出了4种沉积相类型:扇三角洲、辫状河三角洲、湖底扇和湖泊沉积。
3.1.1 扇三角洲
扇三角洲分布在西江36洼和番禺4洼各次洼沉积陡坡带,扇三角洲在地震剖面上楔形特征明显,内部多呈杂乱充填反射(图6-7),局部区域表现为楔形前积地震相;自然伽马形态表现为高幅高频振荡测井相与箱状高值尖峰测井相。高幅高频振荡测井相代表砂泥互层沉积组合,箱状高值尖峰测井相对应厚层砂砾岩夹薄层泥岩沉积组合。从扇三角洲井壁芯来看,其岩性由砾石层与长石石英中粗砂岩组成,单矿物颗粒成分以石英、长石为主,砾石成分以花岗岩和板岩岩屑颗粒等为主,钙泥质胶结,次棱角—棱角状,分选差。砂砾岩夹薄层泥岩中,除含常绿栎粉外,还含有盘星藻、球藻等淡水藻类[5],表明扇三角洲发育时期气候潮湿,扇三角洲已推进到湖盆较深水区(图6)。
3.1.2 辫状河三角洲
辫状河三角洲分布在西江36洼和番禺4洼缓坡带及轴向带。在地震剖面上主要为帚状、叠瓦状或斜交前积反射(图6-7),规模较大的辫状河三角洲复合体分布范围广,其内部显示出多期辫状河三角洲砂体的叠置。从测井曲线形态来看,辫状河三角洲平原亚相以钟形为主,近源部位齿化特征明显,对应辫状水道砂岩向棕褐色泥岩的过渡;辫状河三角洲前缘亚相的测井曲线一般幅度变化较大,以钟形及箱形、漏斗形、指状为主,对应辫状水道砂岩与湖相泥岩互层的沉积组合。根据井壁芯资料,辫状河三角洲砂岩杂基含量比扇三角洲低,属长石质岩屑砂岩、岩屑长石砂岩。砂岩颗粒磨圆度为次圆至次棱状,分选差至中等(图6)。
3.1.3 湖底扇
湖底扇主要为滑塌成因[18-20],与陡坡带粗粒物质相伴生,在研究区内发育于扇三角洲前端,处于洼陷中心内。湖底扇在地震剖面上呈透镜体形态,内部反射杂乱(图6-7)。自然伽马曲线表现为幅度低、振荡频繁、齿化的箱形曲线特征。钻遇典型的湖底扇沉积位于扇三角洲前缘,岩性以细砂岩、粉砂岩为主,层理不发育,多呈块状,有机质含量高,含丰富的浮游藻类[5](图6)。
3.1.4 湖泊沉积
研究区文昌期沉积时期主要发育半深湖—深湖相及滨浅湖相沉积,半深湖—深湖相主要邻近各洼陷的控边断裂;滨湖亚相在地震上多表现为中—低连续亚平行反射结构,而半深湖—深湖相主要表现为席状高—中连续平行地震相(图6-7)。滨浅湖相在湖泊扩展的背景下形成向上变细的沉积序列,在湖泊萎缩的背景下多形成向上变粗的沉积序列。发育波纹层理、波纹交错层理,常见层内冲刷面。测井曲线以中低伽马、中高声波时差为特征,测井曲线呈齿化,有机地化特征表现为低C30-4-甲基甾烷值和较低的T化合物值。而深湖—半深湖是位于湖泊浪基面之下、湖水较为平静的湖区。其沉积物主要为暗色泥岩,具水平层理和块状层理,有机碳含量较高,且富含浮游藻类[5],有机地化特征表现为高C30-4-甲基甾烷值,极低的T化合物值。测井曲线以高伽马、中低声波时差为特征,测井曲线呈微齿化(图6)。
3.2 沉积体系展布和演化
西江36洼和番禺 4 洼文昌组对应裂陷Ⅰ幕沉积,又由T83界面分为下文昌、上文昌两个准二级层序,分别对应裂陷ⅠA幕和裂陷ⅠB幕。裂陷ⅠA幕包括3个三级层序,自下而上对应于文六段、文五段、文四段,裂陷ⅠB幕包括3个层序,自下而上依次为文三段、文二段及文一段。地震相分析表明,陡坡带可识别出楔状杂乱充填地震相和楔状前积地震相,深洼带可识别出席状高—中连续平行地震相以及分布局限的透镜状地震相,斜坡带及轴向带可识别出中—低连续亚平行地震相及前积反射地震相,根据沉积相类型和6个三级层序地震相平面展布特征(图7),结合不同时期的差异构造活动、沉积物供给等条件分析,6个三级层序充填特征及沉积相平面展布各不相同。
3.2.1 裂陷ⅠA幕下文昌沉积展布及演化
裂陷ⅠA幕自下而上包括文六段、文五段、文四段,该时期研究区构造活动强烈,断陷加速沉降,主要发育扇三角洲、辫状河三角洲、湖底扇及湖泊沉积等沉积相类型。
文六段时期,西江36洼边界断层首先开始活动,番禺4洼边界断层还不活动(图4),文六段发育在西江36洼控洼断层下降盘。该时期西江36洼东南部边界断层控洼,在陡坡带边界断裂附近发育小型扇三角洲,深洼带主要发育滨浅湖,缓坡带从西江低凸起到洼陷中心发育辫状河三角洲(图8f)。
文五段时期,西江36洼沉降加剧,番禺4洼主干控洼断层开始活动并活动强烈,番禺4洼南次洼、北次洼及西次洼连片发育,该时期西江36洼与番禺4洼仍未连通,单独存在(图4)。西江36洼及番禺4洼控洼边界断层下降盘发育半深湖—深湖,东沙隆起、西江低凸起及研究区长轴两侧为主要供源,西江36洼与番禺4洼缓坡带及轴向带发育较大规模的辫状河三角洲沉积,同时番禺4洼陡坡带控洼断裂附近发育小规模扇三角洲,在西江36洼及番禺4洼主干控洼断裂附近深洼带发育小范围半深湖—深湖,主要是分布在西江36洼及番禺4洼北次洼,并在番禺4洼半深湖—深湖前端发育舌形湖底扇,滨浅湖较文六段明显扩张(图8e)。
文四段时期,西江36洼及番禺4洼主干控洼断裂活动性最强,水体明显加深,西江36洼与番禺4洼形成统一湖盆,番禺4洼西北次洼也开始出现(图4)。洼陷整体表现为欠补偿环境,相比上个时期,物源方向基本没有变化,但缓坡带稍往物源方向后退,辫状河三角洲规模有所减小,西江36洼与番禺4洼洼陷内陡坡带扇三角洲规模扩大,连片发育;湖盆范围有所扩大的同时,中-深湖范围明显扩大,特别是西江36洼及番禺4洼北次洼,沉积大套黑灰色泥岩,内部可见舌形湖底扇滑塌体(图8d)。
3.2.2 裂陷ⅠB幕上文昌组沉积展布及演化
裂陷ⅠB幕包括3个三级层序,从下而上依次为文三段、文二段、文一段,这个阶段断裂活动同样强烈,凹陷快速沉降,但沉积中心与沉降中心从西江36洼向番禺4洼迁移,具有从东向西迁移特征。该阶段旋回主要发育的沉积相类型与裂陷ⅠA幕相同,但沉积中心、物源方向及规模、相带分布发生明显变化。
文三段时期,两个洼陷在该时期仍为统一湖盆,西江36洼主干边界断裂活动开始减弱,西江36洼湖盆范围变小,番禺4洼整体沉降活动增强,周边控洼断裂皆强烈活动,番禺4洼连成一体,湖盆范围有所扩大(图4)。西江36洼物源方向基本没变化,但缓坡带、轴向带物源向洼陷中心前进,规模扩大;番禺4洼缓坡带及轴向带物源后退,辫状河三角洲规模减小,主要发育陡坡带扇三角洲。西江36洼半深湖—深湖范围相比上个层序减小,番禺4洼受四周控洼断层强烈活动影响,半深湖—深湖范围明显扩大(图8c)。
文二段时期,受西江36洼主干边界断裂活动减弱与番禺4洼边界断裂活动向西部迁移影响,西江36洼与番禺4洼不再是统一湖盆,而是分割为两个独立洼陷(图4)。西江36洼主干边界断裂活动基本不活动,开始萎缩;番禺4洼控洼断裂在南次洼、西次洼及西北次洼活动,湖盆范围减小。西江36洼陡坡带轴向带控洼断裂处出现构造转换带,在陡坡带位置发育从西向东的辫状河三角洲,缓坡带辫状河三角洲不发育;番禺4洼物源方向继承上个时期,规模基本不变,为小型轴向带辫状河三角洲及陡坡带扇三角洲。西江36洼深洼带发育滨浅湖,番禺4洼由于控洼断裂仍在强烈活动,南次洼、西次洼及西北次洼半深湖—深湖仍较发育,在半深湖—深湖泥岩内存在舌形湖底扇滑塌体(图8b)。
文一段时期,西江36洼已经消失,番禺4洼控洼断裂活动性减弱,洼陷范围减小(图4),受后期构造抬升影响发生剥蚀,湖盆残留区陡坡带发育扇三角洲,轴向带发育辫状河三角洲,深洼带主要发育滨浅湖沉积(图8a)。
4. 西江36洼生烃潜力
番禺4洼为“小而肥”富烃洼陷[1-3],而西江36洼却未有商业发现,导致勘探上对西江36洼烃源潜力有所顾虑。通过对不同三级层序时期沉积体系展布研究,西江36洼半深湖—深湖相在文五段至文三段持续发育,继承性发育在NE向边界控洼断裂下降盘附近,并且在下文昌文四段发育面积最大, 由西江36洼供源的砂岩提取烃中发现了较为丰富的C30-4-甲基甾烷,证实了半深湖—深湖相优质烃源岩的存在。传统上洼陷烃源岩潜力评价主要是从有机质生产力、保存条件来开展,然而由于西江36洼未钻遇烃源岩,因而这套方法不适用于该洼陷的生烃潜力评价。
目前近海盆地低勘探程度洼陷的生烃潜力评价主要是利用代表优质烃源岩的半深湖—深湖相发育规模来开展[21],同时笔者通过调研文献认识到半深湖—深湖相有机质的丰度也受到泥岩纯度的影响[22]。本次评价过程中半深湖—深湖相规模用半深湖—深湖面积乘以平均厚度计算,泥岩纯度与物源输入程度有关,物源输入量越大,泥岩纯度越低[22],泥岩纯度用半深湖—深湖周边的物源供给面积乘以平均厚度结果的倒数近似表示。因而西江36洼生烃潜力用半深湖—深湖规模与物源输入量的除值来表征(表1),为了更形象地反映西江36洼生烃潜力,与番禺4洼不同三级层序生烃潜力值进行对比。
表 1 西江36洼与番禺4洼烃源岩条件对比Table 1. Comparison of source rock conditions between Xijiang 36 sag and Panyu 4 sag西江36洼 番禺4洼 层序 中深湖规模 物源供给量 生烃
潜力值中深湖规模 物源供给量 生烃
潜力值面积
/km2平均
厚度/m面积
/km2平均
厚度/m面积
/km2平均
厚度/m面积
/km2平均
厚度/m上文昌 文一段 0 0 − − 0 0 0 − − 0 文二段 0 0 − − 0 180 400 150 250 1.92 文三段 42 450 60 125 2.52 300 600 147 200 6.12 下文昌 文四段 83 650 80 110 6.13 132 600 110 100 7.2 文五段 45 500 70 120 2.68 50 350 80 120 1.82 文六段 0 0 − − 0 0 0 − − 0 注:生烃潜力值=(中深湖面积×中深湖平均厚度)/(物源面积×物源平均厚度)。 文六段时期,西江36洼不发育半深湖—深湖相,烃源岩相带为滨浅湖,番禺4洼还未形成,该时期皆不发育半深湖—深湖相烃源岩,条件一致;文五段时期,面积基本一致,平均厚度更大的情况下,西江36洼半深湖—深湖周边物源供给量更小,西江36洼半深湖—深湖相优质烃源潜力总体好一些;文四段西江36洼与番禺4洼半深湖—深湖皆发育,西江36洼半深湖—深湖相面积为番禺4洼的2/3,但平均厚度更大,同时半深湖—深湖周边物源供给量更小,总的来说该时期西江36洼半深湖—深湖相优质烃源潜力稍微差一些,因而结合文六段至文四段综合情况,下文昌时期两个洼陷的半深湖—深湖相优质烃源岩发育情况基本一致。文三段至文一段,番禺4洼半深湖—深湖相优质烃源岩持续发育,规模较西江36洼大很多,番禺4洼周边物源规模较西江36洼小,因而上文昌时期番禺4洼半深湖—深湖相优质烃源岩条件明显较西江36洼更好。
综合以上分析,番禺4洼文昌组半深湖—深湖相优质烃源岩条件明显比西江36洼更好,同时可以发现在下文昌时期,两个洼陷的半深湖—深湖相优质烃源潜力值基本相同,反映出这个洼陷半深湖—深湖相优质烃源岩发育情况基本一致。根据番禺4洼已探明原油油源分析,半数以上储量来自于下文昌时期半深湖—深湖相烃源岩供烃,因而推测西江36洼下文昌烃源岩也有良好生烃潜力。
5. 结论
(1)西江凹陷南部西江36洼和番禺4洼文昌组作为一个整体可划分为6个三级层序,并且层序发育过程表现为从西江36洼向番禺4洼迁移的特征,西江36洼先形成也先消失,西江36洼发育文六段至文二段等5个三级层序,番禺4洼发育文五段至文一段等5个三级层序。
(2)西江36洼和番禺4洼文昌组发育扇三角洲、辫状河三角洲、湖底扇及湖泊沉积等沉积相类型,垂向上沉积相带具有旋回特征,从初始裂陷到强烈裂陷再到裂陷萎缩,边界断层先变强再变弱,边界主控断层下降盘扇三角洲先变大再变小,缓坡带及轴向带辫状河三角洲先减小后增大,湖盆及半深湖—深湖相规模先增大后减小。横向上沉积相带也具有迁移特征,表现为文六段时期先在西江36洼开始沉积,文五段开始再扩展到番禺4洼,同时文一段时期西江36洼湖盆已经消失,只在番禺4洼有沉积。
(3)通过对比西江36洼与番禺4洼文昌期各三级层序半深湖—深湖相规模及其物源供给量情况,下文昌期西江36洼与番禺4洼烃源潜力基本一致,由于番禺4洼目前半数以上探明原油为下文昌烃源岩提供,因此推测西江36洼下文昌烃源岩具备良好生烃潜力。
-
图 3 研究区珊瑚礁宏观手标本和显微照片
A. 珊瑚骨架灰岩宏观照片;B. 珊瑚骨架灰岩,单偏光;C. 红藻粘结灰岩宏观照片;D. 红藻粘结灰岩,单偏光;E. 生物碎屑灰岩宏观照片;F. 生物屑灰岩,单偏光;G. 含生物碎屑泥灰岩宏观照片;H. 泥晶生屑灰岩,单偏光。
Figure 3. Macroscopic hand specimens and micrographs of coral reefs in the study area
A. Macro photo of coral skeleton limestone; B. Coral skeleton limestone, single polarized light; C. Macro photo of red algae bound limestone; D. Red algae bound limestone, single polarized light; E. Macro photo of bioclastic limestone; F. Bioclastic limestone, single polarized light; G. Macro photo of bioclastic marl; H. Micritic bioclastic limestone, single polarized light.
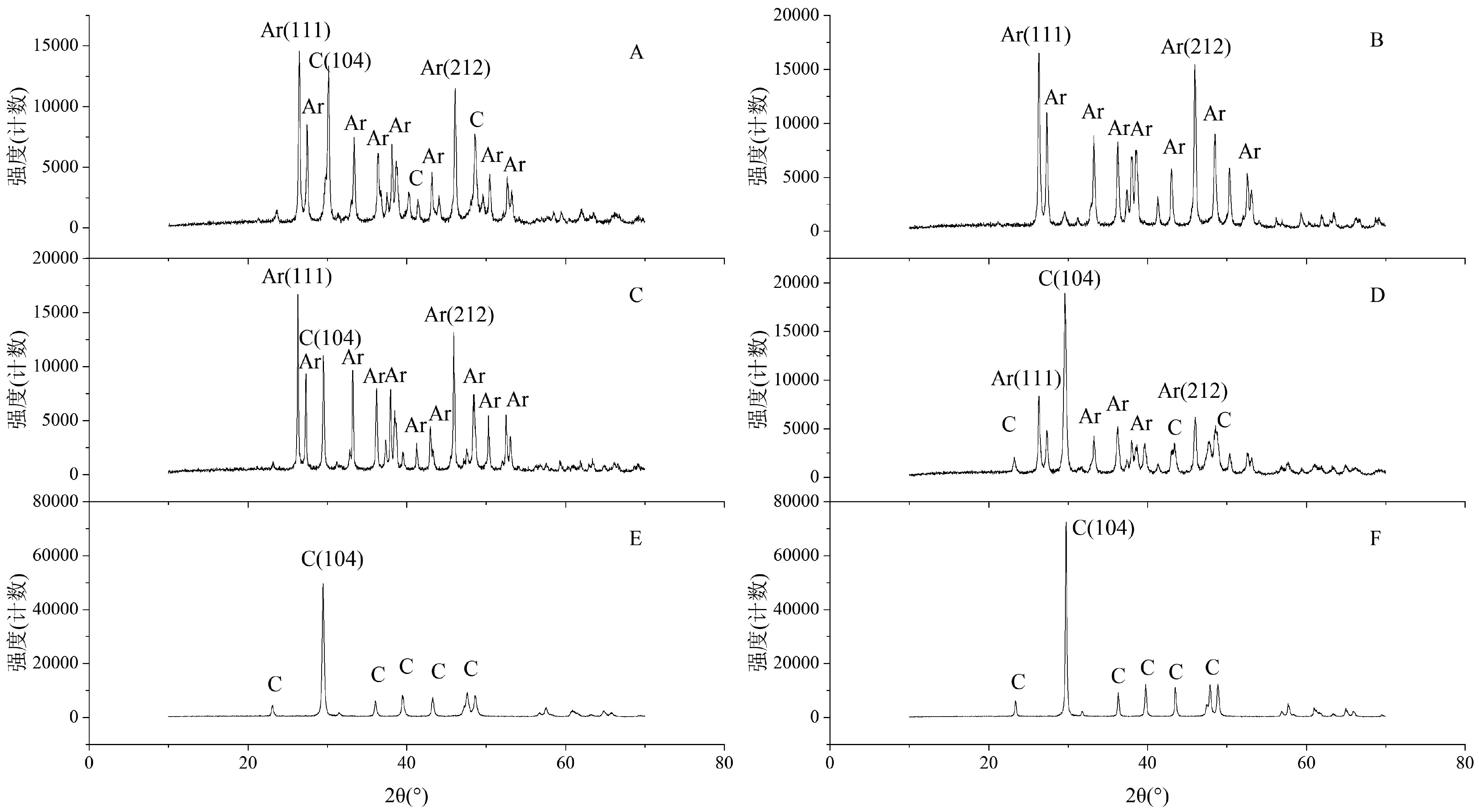
图 4 珊瑚礁样品X射线衍射图谱
Ar. 文石,C. 方解石;A. 18.40 m处生物碎屑灰岩,B. 22.80 m处珊瑚骨架,C. 23 m处滨珊瑚骨骼化石,D. 27.75 m处生物碎屑灰岩,E. 31.20 m处生物碎屑灰岩,F. 41.80 m处红藻黏结灰岩。
Figure 4. X-ray diffraction pattern of coral reef samples
Ar. aragonite, C. calcite. A. Bioclastic limestone at 18.40 m; B. Coral skeleton at 22.80 m; C. Porites skeleton fossil at 23 m; D. Bioclastic limestone at 27.75 m; E. Bioclastic limestone at 31.20 m; F. Red algae bound limestone at 41.80 m.
图 11 滨珊瑚骨骼化石扫描电镜图片
A. 23 m珊瑚骨架(134x),B. 23 m文石(500x),C. 22.80 m珊瑚骨架(989x),D. 22.80 m文石(3.00kx)。
Figure 11. Scanning electron microscope picture of Porites skeleton fossil
A. coral skeleton at 23 m (134x), B. aragonite at 23 m (500X), C. coral skeleton at 22.80 m (989x), D. aragonite at 22.80 m (3.00kx).
图 13 计算了标准化水样和PAAS端元的REY模式
(注:绿色三角为岩心柱数据,红色三角形为滨珊瑚骨骼化石数据,海水端元数据引自文献[49],热液端元数据引自文献[48])
Figure 13. Calculated the REY pattern of standardized water samples and PAAS end elements
(Note: green triangle is core column data, red triangle is Porites skeleton fossil data, seawater end metadata is quoted from reference[49], hydrothermal end metadata is quoted from reference[48])
表 1 珊瑚礁样品矿物物相组成
Table 1 Mineral phase composition of coral reef samples
样品号 深度/m 文石/% 方解石/% 18.40 18.40 76.4 23.6 22.80 22.80 100 − 23 23 76.3 23.7 27.75 27.75 43.6 56.4 31.20 31.20 − 100 41.80 41.80 − 100 注:−表示未检出。 表 2 岩心柱中部分珊瑚(包括滨珊瑚骨骼化石)238U-232Th测年结果
Table 2 238U-232Th dating results of some corals (including
Porites skeleton fossil) in core column 样品号 238U(×10−9) 232Th(×10−12) δ234U*(测量值) 230Th/238U δ234UInitial**(校正后) 年龄/ka 校正后年龄/kaBP YL-1835 1 670±1.1 24 128±52 114±1.1 0.606 4±0.000 745 144±1.4 84.18±0.206 83.82±0.277 YL-1890 2 766±1.8 1 417±44 109±1.0 0.723 8±0.000 900 150±1.3 112.40±0.305 112.38±0.305 YL-2175 2 400±1.3 741±37 109±1.8 0.743 1±0.001 038 151±2.5 117.74±0.473 117.73±0.473 YL-2300 2 480±1.3 398±41 113±1.0 0.734 2±0.000 812 156±1.4 114.36±0.299 114.35±0.30 YL-2495 1 222±0.9 942±46 107±1.2 0.821 4±0.001 177 160±1.8 142.49±0.540 142.47±0.540 YL-3011 3 108±2.2 111 312±143 104±1.0 0.795 9±0.000 981 152±1.4 134.64±0.412 133.73±0.615 YL-3650 1 888±1.2 14 549±45 85±1.1 1.063 0±0.001 193 220±3.8 337.10±3.71 336.92±3.71 YL-4285 924±0.7 6 890±47 89±1.2 1.007 3±0.001 333 183±2.6 255.73±1.837 255.54±1.839 YL-4605 951±0.7 594±40 88±1.1 0.982 6±0.001 357 171±2.2 234.82±1.47 234.80±1.47 YL-4850 963±0.7 579±48 82±1.0 0.986 6±0.001 279 163±2.2 243.85±1.53 243.84±1.53 YL-5015 509±0.4 184±40 92±1.3 0.894 8±0.001 208 152±2.2 177.96±0.848 177.95±0.848 YL-5530 1 816±1.1 158±47 88±1.0 0.962 6±0.001 209 163±1.9 220.19±1.135 220.19±1.135 注:234U、238U和230Th的衰变常数${{\rm{\lambda }}_{{\rm{234}}}}{\rm{ = 2}}.{\rm{82206}} \times {\rm{1}}{{\rm{0}}^{{\rm{ - 6}}}}{{\rm{a}}^{{\rm{ - 1}}}}$、$ {\rm{\lambda }}_{\rm{238}}\rm{=1.551}\rm{}\rm{25} \times {\rm{10}}^{\rm{-10}}{\rm{a}}^{\rm{-1}} $和$ {\rm{\lambda }}_{\rm{234}}\rm{=9.170}\rm{}\rm{5} \times {\rm{10}}^{\rm{-16}}{\rm{a}}^{\rm{-1}} $;$ {\rm{\delta }}^{\rm{234}}\rm{U=}\left({\left[{}_{\rm{}}{}^{\rm{234}}\rm{U}/{}_{\rm{}}{}^{\rm{238}}\rm{U}\right]}_{\rm{activity}}\rm{-1}\right)\times{1}\rm{}\rm{000} $;校正的230Th年龄是假定初始的230Th/232Th原子比为(4.4±2.2)×10−6。年龄均相对于1 950 a。 表 3 主量元素测试结果(单位:%)
Table 3 Major element test results(unit: %)
样品名称 Al2O3 CaO K2O MgO Na2O P2O5 样品名称 SiO2 2-6 0.03 48.62 0.02 0.15 0.53 – 000-1 0.034 2-16 0.03 42.2 0.01 0.12 0.44 0.01 000-2 0.016 5-1 0.01 43.88 0.01 0.17 0.59 – 000-3 0.078 5-8 0.01 43.24 0.01 0.17 0.59 – 000-4 0.031 9-1 0.01 43.6 0.01 0.25 0.59 – 000-5 0.044 9-8 0.01 45.2 0.01 0.24 0.67 – 000-6 0.020 15-1 0.01 43.62 0.01 0.24 0.6 – 001-1 – 15-8 0.01 42.97 0.01 0.24 0.6 – 001-2 0.011 19-1 0.01 52.1 0.02 0.33 0.81 – 001-3 0.057 19-11 0.01 52.4 0.02 0.3 0.99 – 010-1 – 18.75-2 0.01 54.05 – 0.66 0.21 0.08 010-2 – 20.00-1 0.02 53.79 0.01 0.46 0.32 0.05 010-3 – 20.00-2 0.02 53.83 0.01 0.15 0.51 0.01 021-1 – 20.25-1 0.02 63.04 0.01 0.27 0.58 0.01 021-2 0.033 20.25-2 0.01 55.06 0.01 0.45 0.32 0.03 021-3 0.008 21.05-1 0.01 53.09 0.01 0.16 0.53 0.01 034-1 0.004 21.90-2 0.03 57.59 0.01 0.33 0.43 0.04 034-2 0.011 22.75-2 0.01 54.21 – 0.82 0.36 0.05 034-3 0.020 23.40 0.01 54.16 – 0.25 0.27 0.05 039-1 0.015 24.00-1 0.01 53.17 0.01 0.5 0.4 0.02 039-2 0.018 24.00-2 0.01 53.05 – 0.82 0.17 0.04 039-3 – 25.25-1 0.02 53.61 0.01 0.56 0.38 0.05 054-1 0.028 25.25-2 0.02 54.85 – 0.64 0.16 0.04 054-2 0.011 26.55-1 0.01 54.68 – 0.3 0.25 0.02 26.55-2 0.01 54.06 0.01 0.13 0.36 0.01 注:−表示未检出,SiO2为电子探针测试数据。 表 4 稀土元素含量(×10-6)及其相关指标
Table 4 Rare earth element content (×10-6) and related indicators
样品名称 Y La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ΣREY ΣREE ΣLREE/ΣHREE Y/Ho La/La* Ce/Ce* Eu/Eu* BLANK-1 0.000 0.001 0.001 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.002 0.002 13.301 51.776 2.496 0.914 1.549 BLANK-2 0.000 0.000 0.001 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.002 0.001 13.654 259.538 1.743 0.990 1.161 GSR-12 1.311 0.909 2.776 0.257 0.909 0.187 0.039 0.200 0.029 0.168 0.034 0.092 0.012 0.070 0.010 7.003 5.692 8.244 38.690 0.691 1.318 0.952 JDO-1 12.412 7.272 1.890 0.917 3.503 0.611 0.138 0.779 0.106 0.650 0.148 0.420 0.050 0.273 0.038 29.206 16.794 5.815 83.682 1.817 0.161 0.920 2-1 0.096 0.011 0.013 0.002 0.009 0.002 0.001 0.004 0.001 0.004 0.001 0.004 0.001 0.004 0.001 0.153 0.057 2.065 86.474 1.435 0.650 1.928 2-10 0.101 0.011 0.012 0.002 0.008 0.002 0.001 0.004 0.001 0.005 0.001 0.004 0.001 0.004 0.001 0.157 0.055 1.916 89.482 1.574 0.607 1.875 5-8 0.048 0.005 0.010 0.001 0.005 0.001 0.000 0.001 0.000 0.002 0.000 0.002 0.000 0.002 0.000 0.078 0.031 2.699 97.139 1.694 0.972 1.529 9-1 0.064 0.007 0.014 0.001 0.006 0.002 0.001 0.003 0.000 0.003 0.001 0.003 0.000 0.003 0.001 0.109 0.044 2.321 81.114 1.415 1.017 1.336 12-1 0.057 0.006 0.011 0.001 0.005 0.002 0.001 0.002 0.000 0.003 0.001 0.002 0.000 0.002 0.000 0.092 0.035 2.373 96.235 1.168 0.966 2.581 12-7 0.057 0.005 0.011 0.001 0.005 0.001 0.001 0.002 0.000 0.002 0.001 0.002 0.000 0.002 0.000 0.091 0.034 2.538 104.135 1.244 1.010 2.940 22-1 0.059 0.008 0.016 0.002 0.007 0.002 0.001 0.003 0.000 0.002 0.001 0.002 0.000 0.002 0.000 0.106 0.047 3.222 96.631 1.189 0.937 2.347 22-12 0.061 0.006 0.014 0.001 0.006 0.002 0.001 0.002 0.000 0.002 0.001 0.002 0.000 0.002 0.000 0.101 0.040 2.972 105.648 1.163 1.071 2.688 18.60-1 1.306 0.245 0.159 0.047 0.209 0.058 0.015 0.081 0.013 0.092 0.021 0.065 0.009 0.055 0.009 2.383 1.076 2.135 61.970 1.628 0.340 1.021 18.75-2 1.675 0.276 0.189 0.058 0.250 0.071 0.019 0.103 0.016 0.108 0.027 0.082 0.011 0.074 0.012 2.969 1.295 1.992 62.717 1.411 0.346 0.988 18.95-2 2.125 0.327 0.193 0.067 0.305 0.086 0.023 0.125 0.021 0.150 0.035 0.107 0.015 0.090 0.014 3.682 1.557 1.798 60.146 1.571 0.300 1.001 19.25-2 0.240 0.038 0.036 0.008 0.034 0.009 0.003 0.012 0.002 0.013 0.003 0.010 0.001 0.009 0.001 0.419 0.179 2.488 78.458 1.314 0.475 1.348 19.70-1 1.235 0.330 0.277 0.067 0.281 0.070 0.018 0.090 0.014 0.090 0.020 0.061 0.008 0.050 0.008 2.618 1.383 3.064 61.976 1.378 0.429 1.033 19.70-2 1.268 0.366 0.469 0.075 0.309 0.076 0.020 0.096 0.016 0.100 0.022 0.066 0.009 0.057 0.009 2.957 1.690 3.503 56.963 1.278 0.651 1.105 20.00-1 1.159 0.364 0.442 0.074 0.301 0.075 0.022 0.100 0.015 0.093 0.021 0.063 0.009 0.051 0.008 2.798 1.639 3.559 55.897 1.283 0.621 1.191 20.25-1 0.300 0.072 0.053 0.014 0.063 0.017 0.005 0.021 0.003 0.020 0.004 0.013 0.002 0.010 0.002 0.598 0.298 2.958 70.121 1.736 0.387 1.235 20.25-2 0.903 0.204 0.146 0.040 0.179 0.049 0.013 0.064 0.010 0.068 0.015 0.044 0.006 0.035 0.006 1.782 0.879 2.539 59.751 1.609 0.372 1.092 21.05-1 0.127 0.029 0.025 0.004 0.020 0.005 0.002 0.008 0.001 0.008 0.003 0.006 0.001 0.005 0.001 0.246 0.118 2.649 46.176 2.157 0.494 1.300 21.05-2 0.885 0.196 0.140 0.038 0.166 0.045 0.012 0.061 0.010 0.066 0.015 0.043 0.006 0.035 0.005 1.722 0.837 2.484 60.907 1.545 0.372 1.058 21.90-1 0.192 0.055 0.067 0.009 0.036 0.008 0.003 0.011 0.002 0.011 0.002 0.007 0.001 0.006 0.001 0.412 0.219 4.326 78.490 1.357 0.673 1.342 21.90-2 1.202 0.571 0.348 0.093 0.413 0.101 0.025 0.126 0.017 0.111 0.025 0.071 0.010 0.058 0.009 3.179 1.977 3.644 48.811 1.896 0.343 1.029 22.75-2 1.589 0.382 0.252 0.075 0.330 0.088 0.023 0.119 0.018 0.122 0.027 0.078 0.010 0.064 0.010 3.188 1.599 2.565 58.486 1.569 0.343 1.017 23.40 0.681 0.160 0.114 0.033 0.149 0.040 0.011 0.052 0.008 0.050 0.012 0.034 0.005 0.026 0.004 1.379 0.699 2.672 58.194 1.557 0.362 1.068 23.50 0.466 0.131 0.093 0.027 0.126 0.032 0.009 0.041 0.006 0.038 0.009 0.025 0.003 0.019 0.003 1.028 0.562 2.912 54.056 1.657 0.360 1.137 24.00-1 0.760 0.155 0.117 0.030 0.137 0.037 0.010 0.049 0.008 0.053 0.012 0.037 0.005 0.031 0.005 1.446 0.686 2.424 61.510 1.625 0.394 1.067 24.00-2 1.226 0.290 0.208 0.055 0.250 0.068 0.017 0.090 0.014 0.095 0.021 0.063 0.009 0.052 0.008 2.467 1.241 2.527 57.182 1.677 0.378 1.009 25.25-1 1.322 0.303 0.212 0.059 0.263 0.069 0.018 0.092 0.015 0.099 0.023 0.068 0.009 0.055 0.008 2.614 1.292 2.506 58.185 1.560 0.363 1.036 25.25-2 1.160 0.321 0.227 0.064 0.278 0.071 0.018 0.092 0.014 0.094 0.021 0.062 0.008 0.049 0.008 2.487 1.327 2.810 54.668 1.486 0.365 1.017 25.76-1 0.548 0.135 0.093 0.029 0.137 0.038 0.010 0.052 0.008 0.049 0.010 0.029 0.004 0.021 0.003 1.165 0.617 2.508 52.469 1.600 0.341 1.012 25.76-2 1.778 0.339 0.243 0.069 0.305 0.082 0.021 0.110 0.018 0.122 0.029 0.085 0.012 0.070 0.011 3.294 1.516 2.313 62.053 1.502 0.366 1.031 26.55-1 0.376 0.115 0.093 0.024 0.111 0.029 0.009 0.035 0.005 0.033 0.007 0.020 0.003 0.016 0.002 0.880 0.504 3.111 51.114 1.558 0.405 1.350 26.55-2 0.085 0.013 0.016 0.002 0.010 0.003 0.001 0.005 0.001 0.005 0.001 0.003 0.001 0.003 0.000 0.149 0.063 2.560 73.292 2.090 0.717 1.354 27.55-1 2.271 0.718 0.381 0.107 0.485 0.130 0.033 0.174 0.029 0.191 0.043 0.128 0.018 0.101 0.016 4.822 2.551 2.653 52.835 2.161 0.309 1.008 27.55-2 1.549 0.286 0.191 0.056 0.258 0.068 0.018 0.092 0.015 0.105 0.025 0.077 0.011 0.062 0.010 2.822 1.273 2.209 62.010 1.664 0.345 1.032 表 5 微量元素含量(单位:×10−6)
Table 5 Trace element content (unit: ×10−6)
样品名称 Tm Yb Lu Sc Mn Fe Ni Cu Zr Pb U BLANK-1 0.000 0.000 0.000 0.000 0.001 0.179 0.017 0.029 0.006 0.005 0.002 BLANK-2 0.000 0.000 0.000 <LOD 0.002 0.294 0.010 0.013 0.005 <LOD 0.001 GSR-12 0.012 0.070 0.010 0.064 62.169 619.126 74.563 7.958 0.096 1.289 0.074 JDO-1 0.050 0.273 0.038 0.180 51.294 76.914 2.340 0.443 0.118 0.319 0.549 2-1 0.001 0.004 0.001 0.030 2.493 73.733 51.168 82.984 0.029 0.134 1.747 2-10 0.001 0.004 0.001 0.034 0.728 33.919 64.113 3.305 0.022 0.058 1.969 5-8 0.000 0.002 0.000 0.026 0.707 – 1.639 1.041 0.015 0.266 1.804 9-1 0.000 0.003 0.001 0.034 1.765 – 1.553 2.762 0.015 0.262 2.203 12-1 0.000 0.002 0.000 0.038 1.292 1.980 1.149 2.666 0.017 0.084 1.658 12-7 0.000 0.002 0.000 0.039 0.988 1.711 1.100 1.584 0.017 0.119 1.861 22-1 0.000 0.002 0.000 0.033 1.118 2.367 1.049 1.606 0.022 0.114 1.783 22-12 0.000 0.002 0.000 0.032 1.266 3.013 1.533 2.040 0.024 0.112 1.918 18.60-1 0.009 0.055 0.009 0.075 35.654 6.444 9.838 1.059 0.099 0.331 1.725 18.75-2 0.011 0.074 0.012 0.084 23.330 – 3.154 4.937 0.080 0.511 2.285 18.95-2 0.015 0.090 0.014 0.099 30.505 9.423 2.182 0.604 0.139 0.372 1.015 19.25-2 0.001 0.009 0.001 0.051 3.023 2.456 0.796 1.325 0.038 0.065 2.054 19.70-1 0.008 0.050 0.008 0.089 12.991 6.245 3.862 1.797 0.174 0.261 1.383 19.70-2 0.009 0.057 0.009 0.126 12.201 15.666 7.191 2.555 0.651 0.293 1.783 20.00-1 0.009 0.051 0.008 0.112 13.065 – 9.656 0.917 0.582 0.363 2.161 20.25-1 0.002 0.010 0.002 0.045 5.453 3.263 3.261 8.437 0.039 0.155 2.224 20.25-2 0.006 0.035 0.006 0.075 24.240 8.048 1.443 5.293 0.098 0.258 2.112 21.05-1 0.001 0.005 0.001 0.033 2.548 – 5.812 13.849 0.019 0.160 2.780 21.05-2 0.006 0.035 0.005 0.078 13.212 4.427 2.597 13.135 0.057 0.230 1.641 21.90-1 0.001 0.006 0.001 0.046 1.020 4.089 1.174 1.839 0.020 0.042 1.784 21.90-2 0.010 0.058 0.009 0.061 10.913 – 0.688 1.520 0.150 0.788 2.708 22.75-2 0.010 0.064 0.010 0.107 17.480 5.726 0.725 4.369 0.101 0.396 1.723 23.40 0.005 0.026 0.004 0.045 3.529 2.209 0.905 0.478 0.070 0.200 1.974 23.50 0.003 0.019 0.003 0.040 2.536 1.934 0.459 0.630 0.064 0.181 1.964 24.00-1 0.005 0.031 0.005 0.054 5.503 4.197 0.595 3.818 0.065 0.207 1.639 24.00-2 0.009 0.052 0.008 0.072 9.951 5.948 0.720 3.302 0.090 0.343 1.173 25.25-1 0.009 0.055 0.008 0.074 7.101 4.257 2.776 15.709 0.108 0.319 1.627 25.25-2 0.008 0.049 0.008 0.067 8.883 4.157 1.359 16.578 0.102 0.342 1.151 25.76-1 0.004 0.021 0.003 0.050 4.696 2.398 0.536 0.488 0.042 0.146 2.683 25.76-2 0.012 0.070 0.011 0.085 8.654 5.156 0.712 0.768 0.134 0.408 1.966 26.55-1 0.003 0.016 0.002 0.048 3.130 4.850 0.666 0.947 0.048 0.116 1.594 26.55-2 0.001 0.003 0.000 0.028 1.165 – 1.181 0.539 0.039 0.110 2.590 27.55-1 0.018 0.101 0.016 0.109 14.908 5.549 0.726 0.695 0.148 0.514 1.299 27.55-2 0.011 0.062 0.010 0.091 10.275 4.326 0.646 0.883 0.108 0.427 2.101 注:−表示未检测。 -
[1] Kamber B S, Webb G E. The geochemistry of late Archaean microbial carbonate: implications for ocean chemistry and continental erosion history [J]. Geochimica et Cosmochimica Acta, 2001, 65(15): 2509-2525. doi: 10.1016/S0016-7037(01)00613-5
[2] Bolhar R, Van Kranendonk M J, Kamber B S. A trace element study of siderite-jasper banded iron formation in the 3.45 Ga Warrawoona Group, Pilbara Craton-Formation from hydrothermal fluids and shallow seawater [J]. Precambrian Research, 2005, 137(1-2): 93-114. doi: 10.1016/j.precamres.2005.02.001
[3] Bolhar R, Van Kranendonk M J. A non-marine depositional setting for the northern Fortescue Group, Pilbara Craton, inferred from trace element geochemistry of stromatolitic carbonates [J]. Precambrian Research, 2007, 155(3-4): 229-250. doi: 10.1016/j.precamres.2007.02.002
[4] Jiang S Y, Zhao H X, Chen Y Q, et al. Trace and rare earth element geochemistry of phosphate nodules from the lower Cambrian black shale sequence in the Mufu Mountain of Nanjing, Jiangsu province, China [J]. Chemical Geology, 2007, 244(3-4): 584-604. doi: 10.1016/j.chemgeo.2007.07.010
[5] Nothdurft L D, Webb G E, Kamber B S. Rare earth element geochemistry of Late Devonian reefal carbonates, canning basin, Western Australia: confirmation of a seawater REE proxy in ancient limestones [J]. Geochimica et Cosmochimica Acta, 2004, 68(2): 263-283. doi: 10.1016/S0016-7037(03)00422-8
[6] Jiang W, Yu K F, Fan T L, et al. Coral reef carbonate record of the Pliocene-Pleistocene climate transition from an atoll in the South China Sea [J]. Marine Geology, 2019, 411: 88-97. doi: 10.1016/j.margeo.2019.02.006
[7] 赵美霞, 余克服, 张乔民. 珊瑚礁区的生物多样性及其生态功能[J]. 生态学报, 2006, 26(1):186-194. [ZHAO Meixia, YU Kefu, ZHANG Qiaomin. Review on coral reefs biodiversity and ecological function [J]. Acta Ecologica Sinica, 2006, 26(1): 186-194. doi: 10.3321/j.issn:1000-0933.2006.01.025 [8] Fallon S J, White J C, McCulloch M T. <italic>Porites</italic> corals as recorders of mining and environmental impacts: misima Island, Papua New Guinea [J]. Geochimica et Cosmochimica Acta, 2002, 66(1): 45-62. doi: 10.1016/S0016-7037(01)00715-3
[9] Webster J M, Braga J C, Humblet M, et al. Response of the Great Barrier Reef to sea-level and environmental changes over the past 30, 000 years [J]. Nature Geoscience, 2018, 11(6): 426-432. doi: 10.1038/s41561-018-0127-3
[10] 余克服. 南海珊瑚礁及其对全新世环境变化的记录与响应[J]. 中国科学: 地球科学, 2012, 55(8):1217-1229. [YU Kefu. Coral reefs in the South China Sea: their response to and records on past environmental changes [J]. Science China Earth Sciences, 2012, 55(8): 1217-1229. doi: 10.1007/s11430-012-4449-5 [11] Kasper-Zubillaga J J, Armstrong-Altrin J S, Rosales-Hoz L. Geochemical study of coral skeletons from the Puerto Morelos Reef, southeastern Mexico [J]. Estuarine, Coastal and Shelf Science, 2014, 151: 78-87. doi: 10.1016/j.ecss.2014.09.023
[12] Sholkovitz E, Shen G T. The incorporation of rare earth elements in modern coral [J]. Geochimica et Cosmochimica Acta, 1995, 59(13): 2749-2756. doi: 10.1016/0016-7037(95)00170-5
[13] Webb G E, Nothdurft L D, Kamber B S, et al. Rare earth element geochemistry of scleractinian coral skeleton during meteoric diagenesis: a sequence through neomorphism of aragonite to calcite [J]. Sedimentology, 2009, 56(5): 1433-1463. doi: 10.1111/j.1365-3091.2008.01041.x
[14] 陈万利, 吴时国, 黄晓霞, 等. 西沙群岛晚第四纪碳酸盐岩淡水成岩作用——来自永兴岛SSZK1钻孔的地球化学响应证据[J]. 沉积学报, http://doi.org/10.14027/j.issn.1000-0550.2020.006. CHEN WanLi, WU ShiGuo, HUANG XiaoXia, et al. Geochemical signatures in the Late Quaternary meteoric diagenetic carbonate succession, Xisha Islands, South China Sea [J]. Acta Sedimentologica Sinica, http://doi.org/10.14027/j.issn.1000-0550.2020.006.
[15] Zhang R X, Yang S Y. A mathematical model for determining carbon coating thickness and its application in electron probe microanalysis [J]. Microscopy and Microanalysis, 2016, 22(6): 1374-1380. doi: 10.1017/S143192761601182X
[16] Zhang X, Yang S Y, Zhao H, et al. Effect of beam current and diameter on electron probe microanalysis of carbonate minerals [J]. Journal of Earth Science, 2019, 30(4): 834-842. doi: 10.1007/s12583-017-0939-x
[17] 廖泽波, 邵庆丰, 李春华, 等. MC-ICP-MS标样-样品交叉测试法测定石笋样品的<sup>230</sup>Th/U年龄[J]. 质谱学报, 2018, 39(3):295-309. [LIAO Zebo, SHAO Qingfeng, LI Chunhua, et al. Measurement of U/Th Isotopic Compositions in stalagmites for <sup>230</sup>Th/U geochronology using MC-ICP-MS by standard-sample bracketing method [J]. Journal of Chinese Mass Spectrometry Society, 2018, 39(3): 295-309. doi: 10.7538/zpxb.2017.0072 [18] 李晓, 刘娜, 吴仕玖, 等. 南海西沙群岛西科1井上新统-全新统碳酸盐岩微相分析[J]. 科技导报, 2016, 34(7):103-110. [LI Xiao, LIU Na, WU Shijiu, et al. Analysis of carbonate microfacies in Pliocene-Holocene, in Well XK-1, the Xisha Islang, South China Sea [J]. Science & Technology Review, 2016, 34(7): 103-110. doi: 10.3981/j.issn.1000-7857.2016.07.009 [19] 解习农, 谢玉洪, 李绪深, 等. 南海西科1井碳酸盐岩生物礁储层沉积学: 层序地层与沉积演化[M]. 武汉: 中国地质大学出版社, 2016. XIE Xinong, XIE Yuhong, LI Xushen, et al. Sedimentology of carbonate reef reservoirs in Well Xike-1, South China Sea: Sequence Stratigraphy and Sedimentary Evolution[M]. Wuhan: China University of Geosciences, 2016
[20] Van Kranendonk M J, Webb G E, Kamber B S. Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean [J]. Geobiology, 2003, 1(2): 91-108. doi: 10.1046/j.1472-4669.2003.00014.x
[21] Frimmel H E. Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator [J]. Chemical Geology, 2009, 258(3-4): 338-353. doi: 10.1016/j.chemgeo.2008.10.033
[22] Lawrence M G, Greig A, Collerson K D, et al. Rare earth element and yttrium variability in South East Queensland waterways [J]. Aquatic Geochemistry, 2006, 12(1): 39-72. doi: 10.1007/s10498-005-4471-8
[23] Zhao Y Y, Zheng Y F, Chen F K. Trace element and strontium isotope constraints on sedimentary environment of Ediacaran carbonates in southern Anhui, South China [J]. Chemical Geology, 2009, 265(3-4): 345-362. doi: 10.1016/j.chemgeo.2009.04.015
[24] Bayon G, German C R, Burton K W, et al. Sedimentary Fe-Mn oxyhydroxides as paleoceanographic archives and the role of aeolian flux in regulating oceanic dissolved REE [J]. Earth and Planetary Science Letters, 2004, 224(3-4): 477-492. doi: 10.1016/j.jpgl.2004.05.033
[25] Byrne R H, Liu X W, Schijf J. The influence of phosphate coprecipitation on rare earth distributions in natural waters [J]. Geochimica et Cosmochimica Acta, 1996, 60(17): 3341-3346. doi: 10.1016/0016-7037(96)00197-4
[26] Zhao M Y, Zheng Y F. A geochemical framework for retrieving the linked depositional and diagenetic histories of marine carbonates [J]. Earth and Planetary Science Letters, 2017, 460: 213-221. doi: 10.1016/j.jpgl.2016.11.033
[27] Zhao M Y, Zheng Y F. Marine carbonate records of terrigenous input into Paleotethyan seawater: Geochemical constraints from Carboniferous limestones [J]. Geochimica et Cosmochimica Acta, 2014, 141: 508-531. doi: 10.1016/j.gca.2014.07.001
[28] Haley B A, Klinkhammer G P, McManus J. Rare earth elements in pore waters of marine sediments [J]. Geochimica et Cosmochimica Acta, 2004, 68(6): 1265-1279. doi: 10.1016/j.gca.2003.09.012
[29] Bayon G, Birot D, Ruffine L, et al. Evidence for intense REE scavenging at cold seeps from the Niger Delta margin [J]. Earth and Planetary Science Letters, 2011, 312(3-4): 443-452. doi: 10.1016/j.jpgl.2011.10.008
[30] Kidder D L, Krishnaswamy R, Mapes R H. Elemental mobility in phosphatic shales during concretion growth and implications for provenance analysis [J]. Chemical Geology, 2003, 198(3-4): 335-353. doi: 10.1016/S0009-2541(03)00036-6
[31] Kamber B S, Webb G E, Gallagher M. The rare earth element signal in Archaean microbial carbonate: information on ocean redox and biogenicity [J]. Journal of the Geological Society, 2014, 171(6): 745-763. doi: 10.1144/jgs2013-110
[32] Barnard L A, Macintyre I G, Pierce J W. Possible environmental index in tropical reef corals [J]. Nature, 1974, 252(5480): 219-220. doi: 10.1038/252219a0
[33] Porta G D, Webb G E, McDonald I. REE patterns of microbial carbonate and cements from Sinemurian (Lower Jurassic) siliceous sponge mounds (Djebel Bou Dahar, High Atlas, Morocco) [J]. Chemical Geology, 2015, 400: 65-86. doi: 10.1016/j.chemgeo.2015.02.010
[34] Mc Lennan S M, Bock B, Hemming S R, et al. The roles of provenance sedimentary processes in the geochemistry of sedimentary rocks[M]//Lentz D R. Geological Association of Canada Short Course Notes. Toronto: Geological Association of Canada, 2003.
[35] Sholkovitz E R, Piepgras D J, Jacobsen S B. The pore water chemistry of rare earth elements in Buzzards Bay sediments [J]. Geochimica Et Cosmochimica Acta, 1989, 53(11): 2847-2856. doi: 10.1016/0016-7037(89)90162-2
[36] Webb G E, Kamber B S. Rare earth elements in Holocene reefal microbialites: a new shallow seawater proxy [J]. Geochimica Et Cosmochimica Acta, 2000, 64(9): 1557-1565. doi: 10.1016/S0016-7037(99)00400-7
[37] Banner J L, Hanson G N, Meyers W J. Rare earth element and nd isotopic variations in regionally extensive dolomites from the burlington-keokuk formation (Mississippian): implications for REE mobility during carbonate diagenesis [J]. Journal of Sedimentary Research, 1988, 58(3): 415-432.
[38] Kim J H, Torres M E, Haley B A, et al. The effect of diagenesis and fluid migration on rare earth element distribution in pore fluids of the northern Cascadia accretionary margin [J]. Chemical Geology, 2012, 291: 152-165. doi: 10.1016/j.chemgeo.2011.10.010
[39] Shields G, Stille P. Diagenetic constraints on the use of cerium anomalies as palaeoseawater redox proxies: an isotopic and REE study of Cambrian phosphorites [J]. Chemical Geology, 2001, 175(1-2): 29-48. doi: 10.1016/S0009-2541(00)00362-4
[40] Bau M, Koschinsky A, Dulski P, et al. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater [J]. Geochimica et Cosmochimica Acta, 1996, 60(10): 1709-1725. doi: 10.1016/0016-7037(96)00063-4
[41] Shields G A, Webb G E. Has the REE composition of seawater changed over geological time? [J]. Chemical Geology, 2004, 204(1-2): 103-107. doi: 10.1016/j.chemgeo.2003.09.010
[42] Bau M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect [J]. Contributions to Mineralogy and Petrology, 1996, 123(3): 323-333. doi: 10.1007/s004100050159
[43] Bau M, Dulski P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa [J]. Precambrian Research, 1996, 79(1-2): 37-55. doi: 10.1016/0301-9268(95)00087-9
[44] Tanaka K, Tani Y, Takahashi Y, et al. A specific Ce oxidation process during sorption of rare earth elements on biogenic Mn oxide produced by <italic>Acremonium</italic> sp. strain KR21-2 [J]. Geochimica et Cosmochimica Acta, 2010, 74(19): 5463-5477. doi: 10.1016/j.gca.2010.07.010
[45] German C R, Elderfield H. Application of the Ce anomaly as a paleoredox indicator: the ground rules [J]. Paleoceanography, 1990, 5(5): 823-833. doi: 10.1029/PA005i005p00823
[46] Ling H F, Chen X, Li D, et al. Cerium anomaly variations in Ediacaran-earliest Cambrian carbonates from the Yangtze Gorges area, South China: implications for oxygenation of coeval shallow seawater [J]. Precambrian Research, 2013, 225: 110-127. doi: 10.1016/j.precamres.2011.10.011
[47] Kawabe I, Kitahara Y, Naito K. Non-chondritic yttrium/holmium ratio and lanthanide tetrad effect observed in pre-Cenozoic limestones [J]. Geochemical Journal, 1991, 25(1): 31-44. doi: 10.2343/geochemj.25.31
[48] Bau M, Dulski P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater [J]. Chemical Geology, 1999, 155(1-2): 77-90. doi: 10.1016/S0009-2541(98)00142-9
[49] Alibo D S, Nozaki Y. Rare earth elements in seawater: Particle association, shale-normalization, and Ce oxidation [J]. Geochimica et Cosmochimica Acta, 1999, 63(3-4): 363-372. doi: 10.1016/S0016-7037(98)00279-8
[50] Luong L D, Ryuichi S, Nguyen H, et al. Spatial variations in dissolved rare earth element concentrations in the East China Sea water column [J]. Marine Chemistry, 2018, 205: 1-15. doi: 10.1016/j.marchem.2018.07.004
[51] Michard A, Albarède F, Michard G, et al. Rare-earth elements and uranium in high-temperature solutions from East Pacific Rise hydrothermal vent field (13°N) [J]. Nature, 1983, 303(5920): 795-797. doi: 10.1038/303795a0
[52] German C R, Klinkhammer G P, Edmond J M, et al. Hydrothermal scavenging of rare-earth elements in the ocean [J]. Nature, 1990, 345(6275): 516-518. doi: 10.1038/345516a0
[53] Chen D Z, Qing H R, Yan X, et al. Hydrothermal venting and basin evolution (Devonian, South China): constraints from rare earth element geochemistry of chert [J]. Sedimentary Geology, 2006, 183(3-4): 203-216. doi: 10.1016/j.sedgeo.2005.09.020
[54] Kamber B S, Greig A, Collerson K D. A new estimate for the composition of weathered young upper continental crust from alluvial sediments, Queensland, Australia [J]. Geochimica et Cosmochimica Acta, 2005, 69(4): 1041-1058. doi: 10.1016/j.gca.2004.08.020
[55] Wang Q X, Lin Z J, Chen D F. Geochemical constraints on the origin of Doushantuo cap carbonates in the Yangtze Gorges area, South China [J]. Sedimentary Geology, 2014, 304: 59-70. doi: 10.1016/j.sedgeo.2014.02.006
[56] Michard A, Albarède F. The REE content of some hydrothermal fluids [J]. Chemical Geology, 1986, 55(1-2): 51-60. doi: 10.1016/0009-2541(86)90127-0
[57] Alexander B W, Bau M, Andersson P, et al. Continentally-derived solutes in shallow Archean seawater: rare earth element and Nd isotope evidence in iron formation from the 2.9 Ga Pongola Supergroup, South Africa [J]. Geochimica et Cosmochimica Acta, 2008, 72(2): 378-394. doi: 10.1016/j.gca.2007.10.028
[58] Robbins L J, Lalonde S V, Planavsky N J, et al. Trace elements at the intersection of marine biological and geochemical evolution [J]. Earth-Science Reviews, 2016, 163: 323-348. doi: 10.1016/j.earscirev.2016.10.013
[59] Bau M, Balan S, Schmidt K, et al. Rare earth elements in mussel shells of the <italic>Mytilidae</italic> family as tracers for hidden and fossil high-temperature hydrothermal systems [J]. Earth and Planetary Science Letters, 2010, 299(3-4): 310-316. doi: 10.1016/j.jpgl.2010.09.011
[60] Johannessen K C, Roost J V, Dahle H, et al. Environmental controls on biomineralization and Fe-mound formation in a low-temperature hydrothermal system at the Jan Mayen Vent Fields [J]. Geochimica et Cosmochimica Acta, 2017, 202: 101-123. doi: 10.1016/j.gca.2016.12.016
[61] Ho K S, Chen J C, Juang W S. Geochronology and geochemistry of late Cenozoic basalts from the Leiqiong area, Southern China [J]. Journal of Asian Earth Sciences, 2000, 18(3): 307-324. doi: 10.1016/S1367-9120(99)00059-0
[62] 孙嘉诗. 南海北部及广东沿海新生代火山活动[J]. 海洋地质与第四纪地质, 1991, 11(3):45-66. [SUN Jiashi. Cenozoic volcanic activity in the Northern South China Sea and Guangdong coastal area [J]. Marine Geology & Quaternary Geology, 1991, 11(3): 45-66. [63] 樊祺诚, 孙谦, 李霓, 等. 琼北火山活动分期与全新世岩浆演化[J]. 岩石学报, 2004, 20(3):533-544. [FAN Qicheng, SUN Qian, LI Ni, et al. Periods of volcanic activity and magma evolution of Holocene in North Hainan Island [J]. Acta Petrologica Sinica, 2004, 20(3): 533-544. doi: 10.3969/j.issn.1000-0569.2004.03.017 [64] 冯英辞, 詹文欢, 孙杰, 等. 西沙海域上新世以来火山特征及其形成机制[J]. 热带海洋学报, 2017, 36(3):73-79. [FENG Yingci, ZHAN Wenhuan, SUN Jie, et al. The formation mechanism and characteristics of volcanoes in the Xisha waters since Pliocene [J]. Journal of Tropical Oceanography, 2017, 36(3): 73-79. [65] 邹和平. 试谈南海海盆地壳属性问题—由南海海盆及其邻区玄武岩的比较研究进行讨论[J]. 大地构造与成矿学, 1993, 17(4):293-303. [ZOU Heping. On the problem about the crust’s attribution of South China Sea basin-discussion from comparative study on basalts of seamounts in South China Sea basin and the neighboring areas [J]. Geotectonica et Metallogenia, 1993, 17(4): 293-303. [66] 吕炳全, 王国忠, 全松青, 等. 试论西沙群岛石岛的形成[J]. 地质科学, 1986(1):82-89. [LV Bingquan, WANG Guozhong, QUAN Songqing, et al. A preliminary study of the formation of Shidao Island, Xisha Islands [J]. Chinese Journal of Geology, 1986(1): 82-89. -
期刊类型引用(7)
1. 刘帆,廖慧鸿,梅安鑫,梁茹,彭宇. 应用自然伽马能谱法恢复碳酸盐岩沉积时的古水深——以山西兴县关家崖马五_5亚段为例. 中国地质调查. 2024(01): 57-64 .  百度学术
百度学术
2. 梁金同,周刚,曾云贤,和源,朱华,罗冰,谢忱,刘四兵,李笑天,张浩,文华国. 基于自然伽马能谱测井资料的碳酸盐岩层序地层划分及其对古环境恢复的指示意义——以川东北LT1井下寒武统龙王庙组为例. 地质论评. 2023(02): 448-460 .  百度学术
百度学术
3. 曹子颜,徐国盛,颜瑞晶. 黔北坳陷北缘寒武系娄山关群地球化学特征及古海洋环境恢复. 天然气地球科学. 2023(09): 1652-1665 .  百度学术
百度学术
4. 李斌,董振国,罗群. 自然伽马能谱测井在海相页岩储层评价中的应用研究. 核电子学与探测技术. 2023(03): 604-614 .  百度学术
百度学术
5. 叶涛,王清斌,代黎明,陈容涛,崔普媛. 台地相碳酸盐岩层序划分新方法——以渤中凹陷奥陶系为例. 岩性油气藏. 2021(03): 95-103 .  百度学术
百度学术
6. 曹庆超,白壮壮,李浩武,张宁宁. 测井数据小波变换在川中寒武系洗象池群层序地层划分中的应用. 地质科技通报. 2021(04): 242-251 .  百度学术
百度学术
7. 李峰峰,郭睿,余义常. 层序地层划分方法进展及展望. 地质科技情报. 2019(04): 215-224 .  百度学术
百度学术
其他类型引用(8)




 下载:
下载: