Variation and influencing factors of δ13C and δ18O in the inner and outer layers of modern Tridacna squamosa from the Xisha Islands, South China Sea
-
摘要:
砗磲具有清晰的生长纹层,是记录热带海洋气候变化的良好载体,其壳体δ18O、δ13C已广泛应用于第四纪古气候研究。然而,目前大多数研究主要集中在内层壳体,关于外层壳体的研究十分稀少。本研究通过对采自南海西沙群岛浪花礁一个现代鳞砗磲的内外层壳体进行月分辨率δ18O、δ13C测试和内壳日生长纹层扫描,分析内外层壳体δ18O和δ13C的变化特征及其影响因素。结果显示该砗磲外壳δ18O比内壳δ18O更加偏正,但是两者都具有十分相似的变化特征,主要受到海表面温度的控制,表明砗磲内外层壳体δ18O能够可靠地指示气候环境的变化。砗磲内外层壳体δ13C存在较大的差异,其中内壳δ13C具有明显的年周期变化。通过对比分析砗磲内壳δ13C与日生长速率、气候环境参数,发现内壳δ13C的季节变化主要与初级生产力和砗磲自身生命活动有关。砗磲外壳δ13C相对于内壳δ13C较为偏负,表现出持续下降的趋势,且无季节性变化,这可能是由采样路径偏离最大生长轴导致的。
Abstract:Tridacna shells, known for their distinct growth bands, serve as excellent proxies for recording tropical marine climate changes. The δ18O and δ13C in Tridacna shells have been applied in the Quaternary paleoclimate research. However, most previous researches focused on the inner layer, while the outer layer received less attention. We conducted a comprehensive analysis at a monthly resolution δ18O, δ13C tests on both the inner and outer layers, as well as daily growth band scans on the inner layer of modern Tridacna squamosa from the Xisha Islands in the South China Sea. Results reveal that the outer layer exhibited higher δ18O values than the inner layer. In addition, both shells displayed similar variation patterns, being primarily influenced by sea surface temperature (SST), and indicating that the δ18O of the outer layer could reliably indicate climate and environmental changes. In contrast, significant differences in δ13C value were observed between the inner and outer layers. The inner layer displayed a noticeable annual cycle in δ13C value. By comparing the inner layer δ13C, climatic and environmental parameters, and daily growth rate (DGR), we determined that the seasonal variations in inner layer δ13C were linked to the primary productivity and the life activities of Tridacna. Meanwhile, the δ13C of the outer layer, which is relatively negative compared to the inner layer, exhibited a continuous downward trend without seasonal changes. This discrepancy may be attributed to the sampling path deviating from the maximum growth axis.
-
Keywords:
- Tridacna /
- δ13C /
- δ18O /
- climate change /
- South China Sea
-
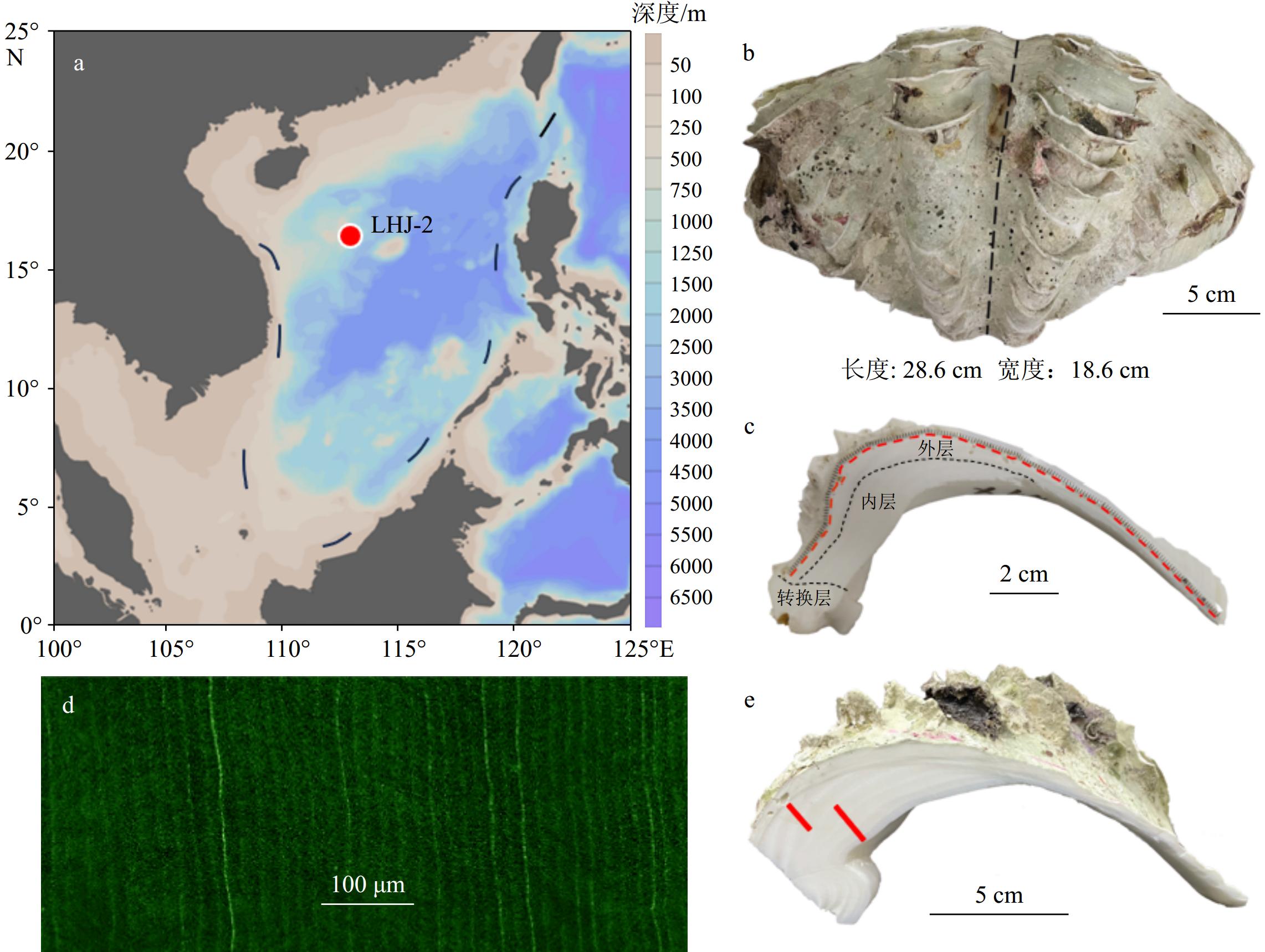
图 1 砗磲样品及其地理位置
a:浪花礁位置示意图(红点)(图片来源:Ocean Data View);b:现代鳞砗磲LHJ-2壳体(黑色虚线为壳体最大生长轴);c:外壳取样路径(红色虚线),黑色虚线为内层、外层和转换层的分界线;d:内壳日生长纹层,e:内壳取样路径(红色实线)。
Figure 1. Tridacna samples and the geographical location
a: A schematic diagram of the location of the Bombay Reef (red dot) (Photo source: Ocean Data View); b: the modern Tridacna shell LHJ-2 (the black dotted line represents the maximum growth axis of the shell); c: outer layer sampling path (red dotted line). The black dotted line is the boundary between the inner layer and the hinge and outer layer; d: daily growth layer of the inner layer; e: inner layer sampling path (red solid line).
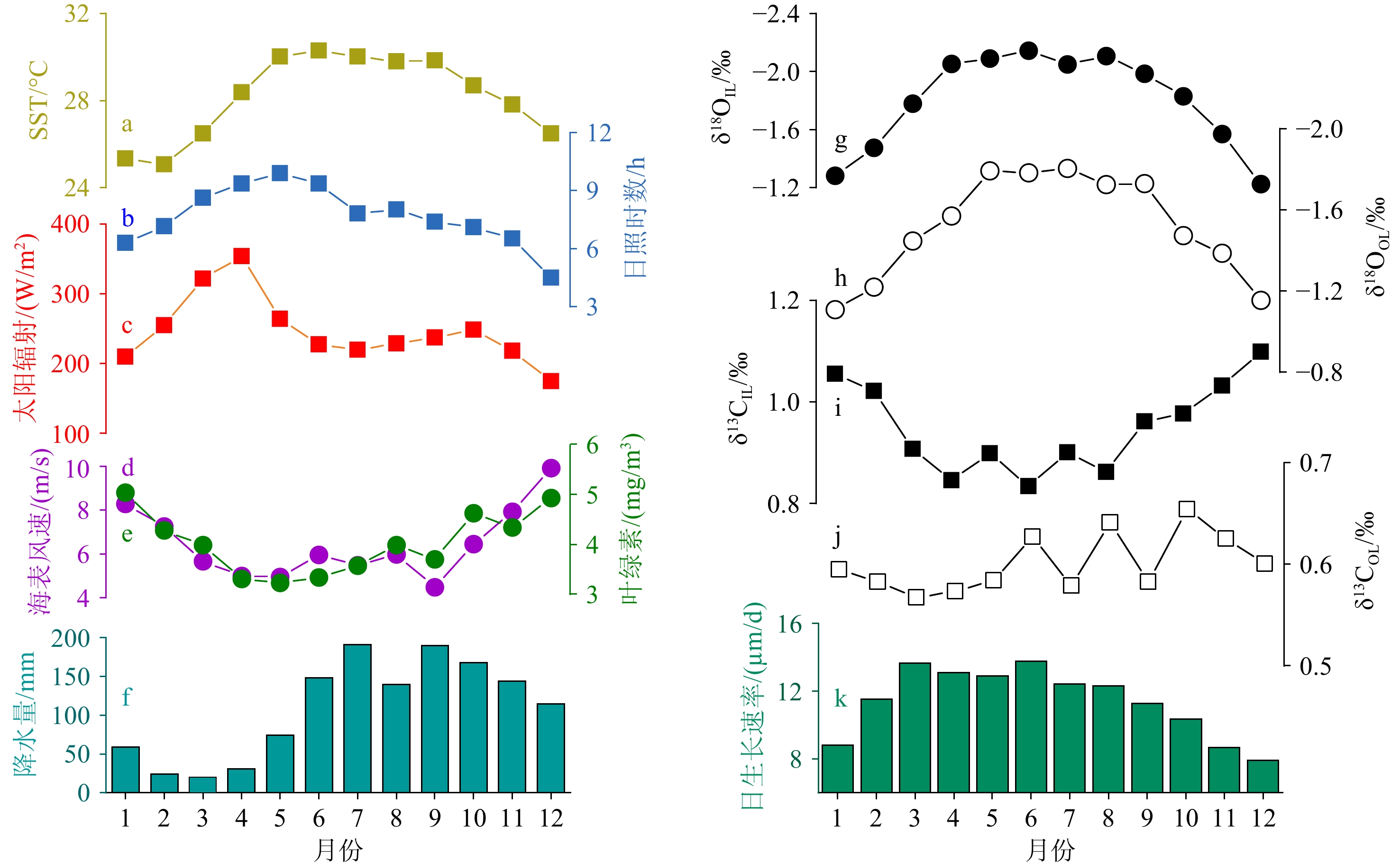
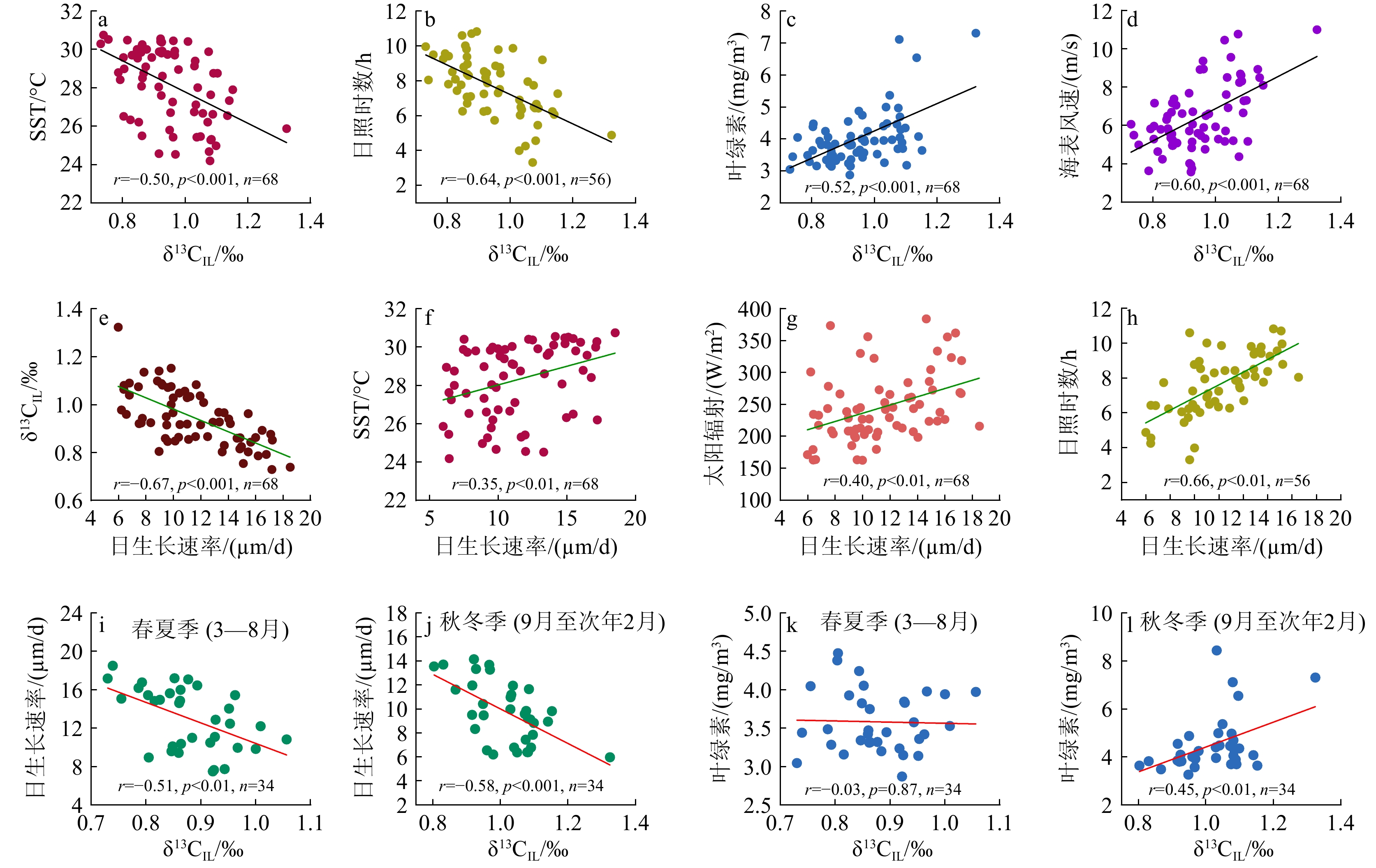
图 2 2013—2018年砗磲的月平均碳、氧同位素记录、日生长速率与当地气候环境参数对比
a:海表面温度,b:日照时数,c:太阳辐射,d:海表风速,e:叶绿素浓度,f:降水量,g:内壳δ18OIL,h:外壳δ18OOL,i:内壳δ13CIL,j:外壳δ13COL,k:内壳月均日生长速率。
Figure 2. Comparison among the monthly average carbon and oxygen isotope records, daily growth rate of Tridacna, and local environmental parameters from 2013 to 2018
a: Sea surface temperature, b: sunlight durations, c: solar radiation, d:sea surface wind speed, e: chlorophyll concentration, f: rainfall, g: inner layer δ18OIL, h: outer layer δ18OOL, i: inner layer δ13CIL, j: outer layer δ13COL, k: average daily growth rate of the inner layer in every month.
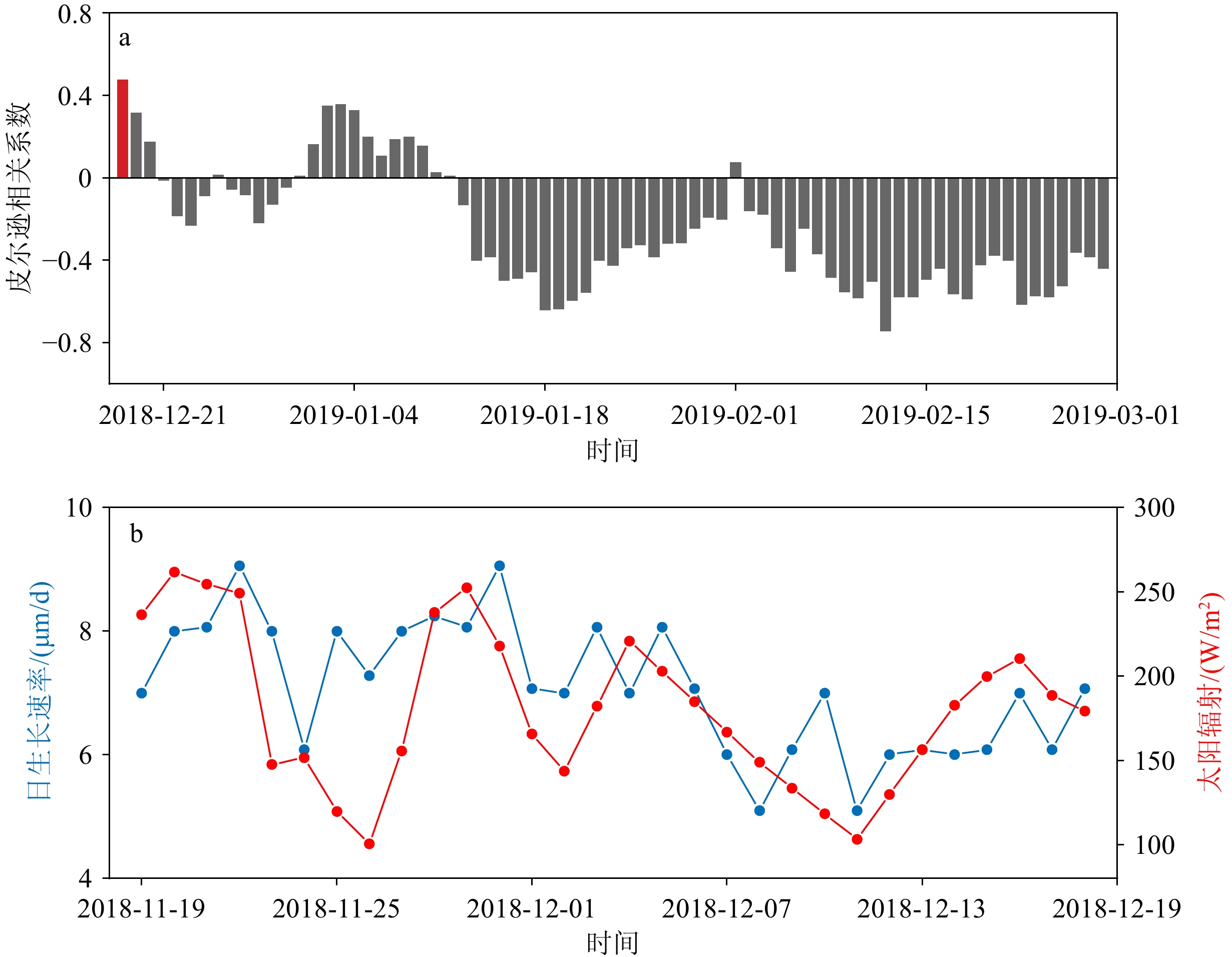
图 3 砗磲日生长速率(DGR)和太阳辐射的关系
a:2018-12-18—2019-2-28的太阳辐射与最后30个DGR的滑动平均相关性分析,红色条形柱代表的日期为2018-12-18,相关系数为 r = 0.47, p < 0.5, n = 73;b: 2018-11-19—2018-12-18的太阳辐射和DGR的对比。
Figure 3. The relationship between the daily growth rate (DGR) of Tridacna and solar radiation
a: The sliding correlation analysis of the last 30 values of DGR and corresponded solar radiation from 18 December 2018 to 28 February 2019. The date represented by the red bar column is 18 December 2018, and the correlation coefficients are r = 0.47, p<0.5, n = 73; b: comparison between solar radiation and DGR from 19 November 2018 to 18 December 2018.
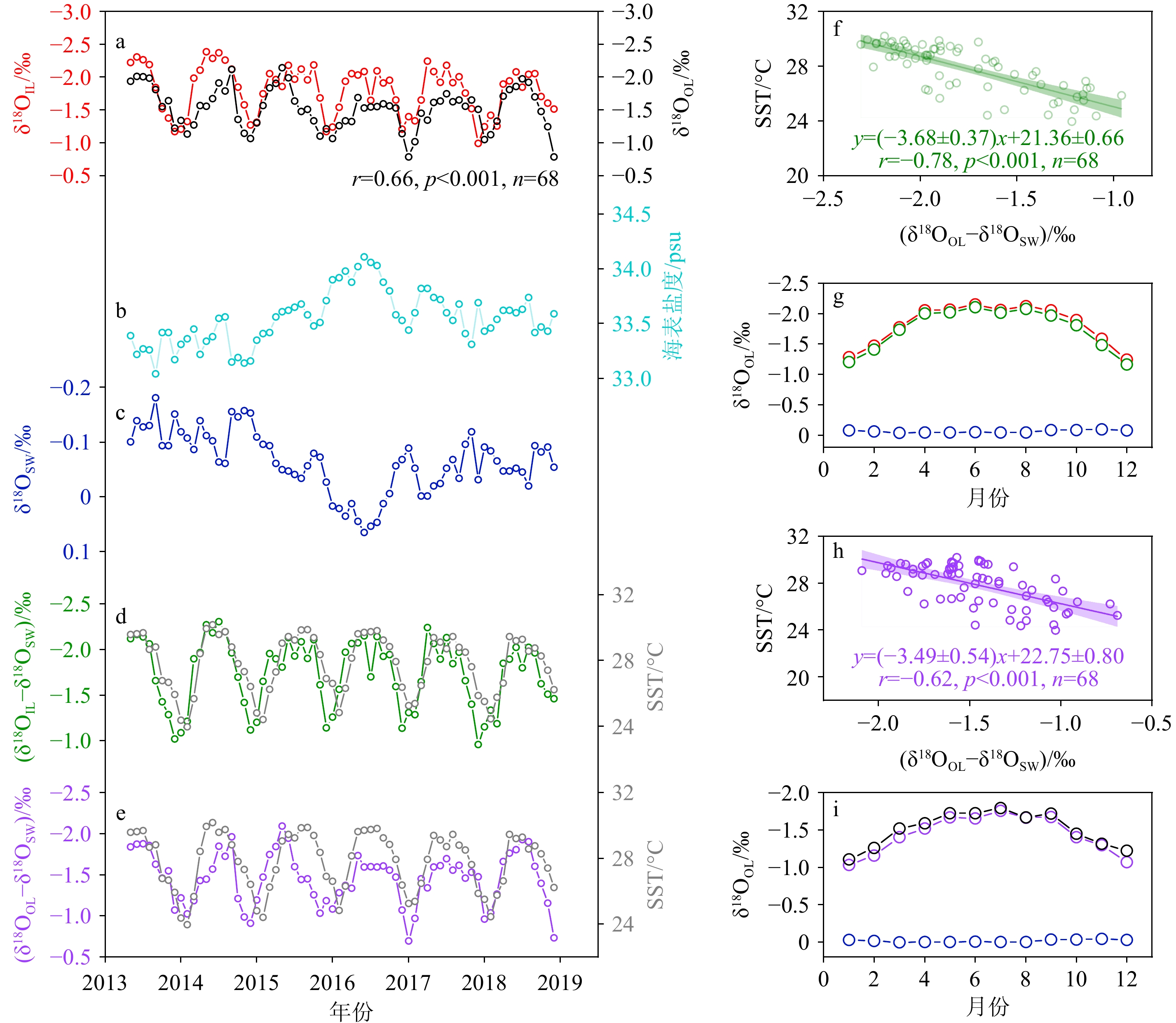
图 4 样品LHJ-2 内外层壳体δ18O、SST以及δ18OSW之间的关系
a:内壳δ18OIL(红色圆圈)和外壳δ18OOL(黑色圆圈),b:海表盐度,c:海水δ18OSW,d:(δ18OIL−δ18OSW)(绿色圆圈)、SST(灰色圆圈),e:(δ18OOL−δ18OSW)(紫色圆圈),SST(灰色圆圈),f:SST与(δ18OIL−δ18OSW)的线性关系,g: 2013—2018年月平均δ18OIL(红色圆圈)、(δ18OIL−δ18OSW)(绿色圆圈)和δ18OSW(蓝色圆圈),h: SST与(δ18OOL−δ18OSW)的线性关系,i: 2013—2018年月平均δ18OOL(黑色圆圈)、(δ18OOL−δ18OSW)(紫色圆圈)和δ18OSW(蓝色圆圈)。
Figure 4. The relationship among δ18O, SST and δ18OSW in the inner and outer layers of the sample LHJ-2
a: Inner layer δ18OIL (red circle) and outer layer δ18OOL (black circle), b: sea surface salinity, c: seawater δ18OSW, d: (δ18OIL−δ18OSW) (green circle), SST(grey circle), e: (δ18OOL−δ18OSW) (purple circle), SST(grey circle), f: the linear relationship between SST and (δ18OIL−δ18OSW), g: the monthly average of δ18OIL (red circle), (δ18OIL−δ18OSW) (green circle) and δ18OSW (blue circle) from 2013 to 2018, h: the linear relationship between SST and (δ18OOL−δ18OSW), i: the monthly average of δ18OOL (black circle), (δ18OOL−δ18OSW) (purple circle) and δ18OSW (blue circle) from 2013 to 2018.
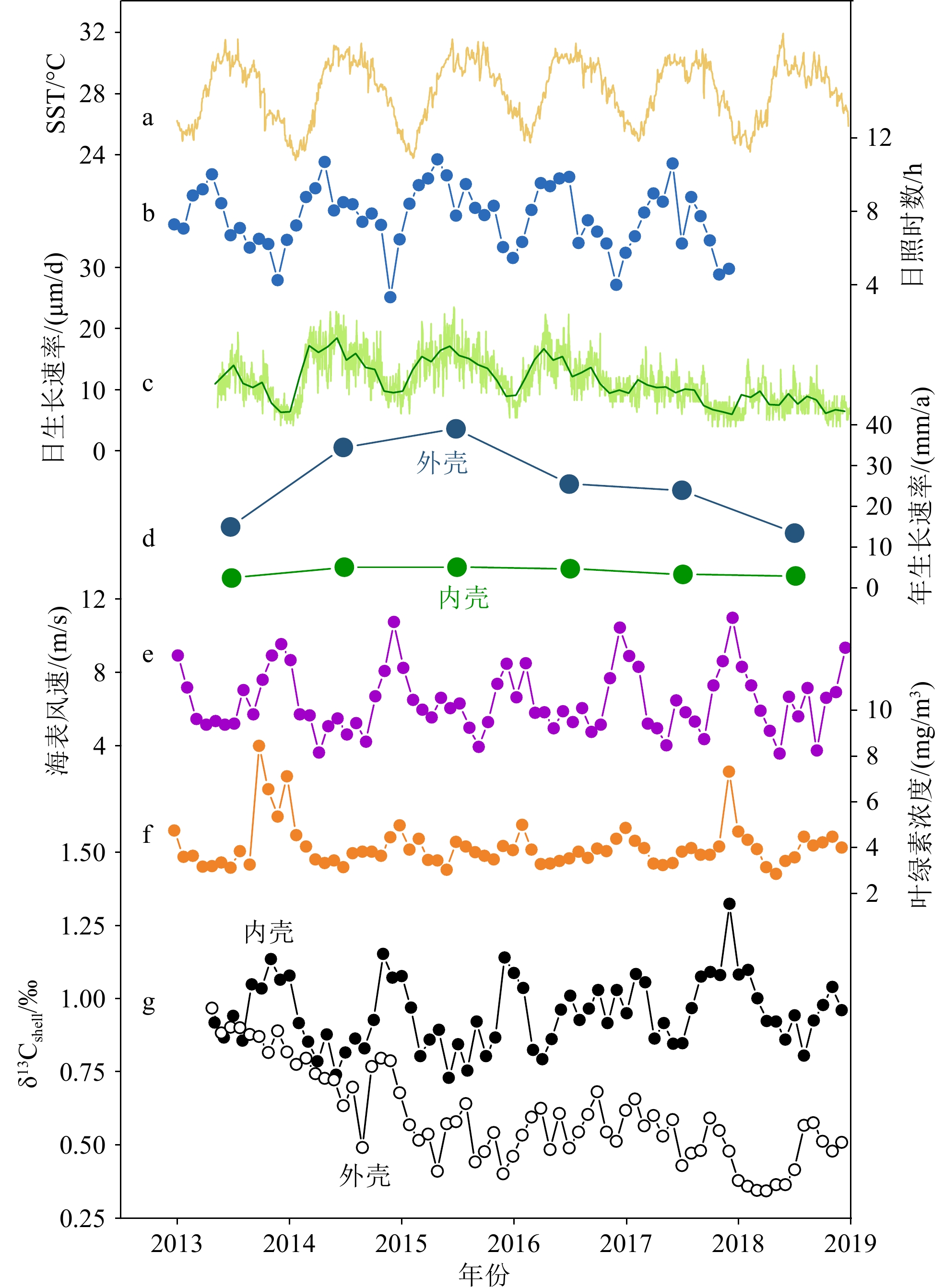
图 5 2013—2018年西沙群岛环境参数与样品LHJ-2壳体δ13C的对比
a: SST,b:日照时数,c:内壳日生长速率,d:内外层壳体年生长速率,e:海表风速,f:叶绿素浓度,g: 内壳δ13C(黑色实心圆圈)、外壳δ13C(黑色空心圆圈)。
Figure 5. The comparison of environmental parameters with δ13C of LHJ-2 shell in Xisha Islands from 2013 to 2018
a: SST, b: sunlight durations, c: the daily growth rate of the inner layer, d: the annual growth rate of the inner and outer layers, e: sea surface wind speed, f: chlorophyll concentration, g: the δ13C of inner layer (black solid circle), the δ13C of outer layer (black hollow circle).
图 6 样品LHJ-2内壳δ13CIL、日生长速率以及各环境参数之间的相关性
a: δ13CIL与SST,b: δ13CIL与日照时数,c: δ13CIL与叶绿素浓度,d: δ13CIL与海表风速,e: δ13CIL与日生长速率,f:日生长速率与SST,g: 日生长速率与太阳辐射,h: 日生长速率与日照时数,i:春夏季的δ13CIL与日生长速率,j:秋冬季的δ13CIL与日生长速率,k:春夏季的δ13CIL与叶绿素浓度,l:秋冬季的δ13CIL与叶绿素浓度。
Figure 6. The correlations of δ13CIL and daily growth rate of the LHJ-2 inner layer to the environmental parameters
a:δ13CIL and SST, b: δ13CIL and sunlight durations, c: δ13CIL and chlorophyll concentration, d: δ13CIL and sea surface wind speed, e: DGR and δ13CIL, f: DGR and SST, g: DGR and solar radiation, h: DGR and sunlight durations, i: δ13CIL and DGR in spring and summer, j: δ13CIL and DGR in autumn and winter, k: δ13CIL and chlorophyll concentration in spring and summer, l: δ13CIL and chlorophyll concentration in autumn and winter.
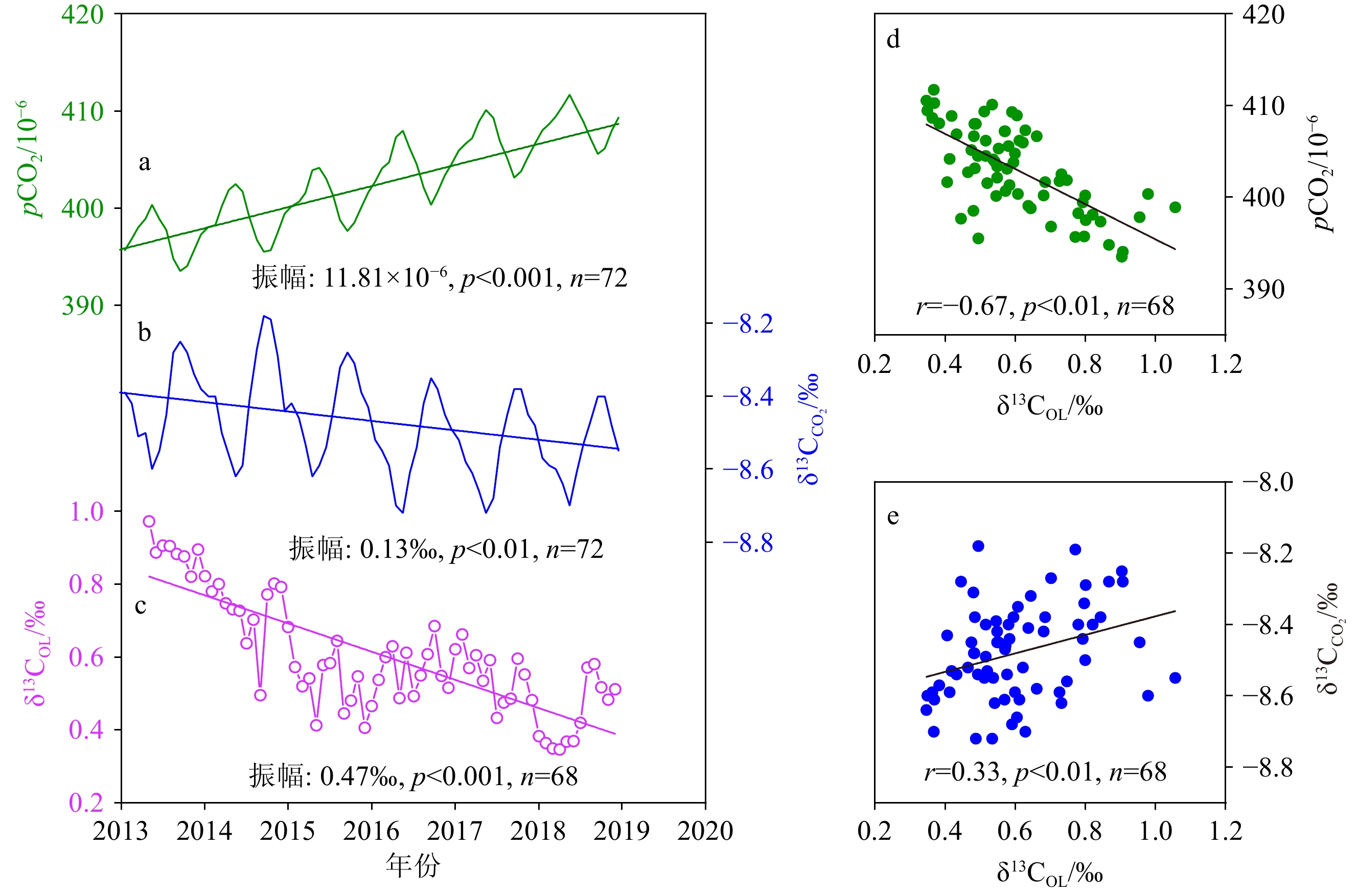
图 7 样品LHJ-2外壳δ13COL与pCO2及其δ13C的对比
a:大气CO2浓度,b:大气CO2的δ13C,c: δ13COL,d: δ13COL与pCO2的相关性,e: δ13COL与大气CO2的δ13C的相关性。
Figure 7. Comparison among δ13COL in the LHJ-2 outer layer, pCO2, and δ13C of atmosphere
a: pCO2, b: δ13C of atmospheric CO2, c: δ13COL, d: the correlation between δ13COL and pCO2, e: the correlation between δ13COL and δ13C in atmospheric CO2.
-
[1] Bonham K. Growth rate of giant clam Tridacna gigas at Bikini Atoll as revealed by radioautography[J]. Science, 1965, 149(3681):300-302. doi: 10.1126/science.149.3681.300
[2] Rosewater J. The family tridacnidae in the Indo-Pacific[J]. Indo-Pacific Mollusca, 1965, 1:347-396.
[3] Neo M L, Eckman W, Vicentuan K, et al. The ecological significance of giant clams in coral reef ecosystems[J]. Biological Conservation, 2015, 181:111-123. doi: 10.1016/j.biocon.2014.11.004
[4] Watanabe T, Suzuki A, Kawahata H, et al. A 60-year isotopic record from a mid-Holocene fossil giant clam (Tridacna gigas) in the Ryukyu Islands: physiological and paleoclimatic implications[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2004, 212(3-4):343-354. doi: 10.1016/S0031-0182(04)00358-X
[5] Elliot M, Welsh K, Chilcott C, et al. Profiles of trace elements and stable isotopes derived from giant long-lived Tridacna gigas bivalves: potential applications in paleoclimate studies[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2009, 280(1-2):132-142. doi: 10.1016/j.palaeo.2009.06.007
[6] Yan H, Shao D, Wang Y H, et al. Sr/Ca profile of long-lived Tridacna gigas bivalves from South China Sea: a new high-resolution SST proxy[J]. Geochimica et Cosmochimica Acta, 2013, 112:52-65. doi: 10.1016/j.gca.2013.03.007
[7] Ayling B F, Chappell J, Gagan M K, et al. ENSO variability during MIS 11 (424–374 ka) from Tridacna gigas at Huon Peninsula, Papua New Guinea[J]. Earth and Planetary Science Letters, 2015, 431:236-246. doi: 10.1016/j.jpgl.2015.09.037
[8] Gannon M E, Pérez-Huerta A, Aharon P, et al. A biomineralization study of the Indo-Pacific giant clam Tridacna gigas[J]. Coral Reefs, 2017, 36(2):503-517. doi: 10.1007/s00338-016-1538-5
[9] Yan H. Daily growth bands of giant clam shell: a potential paleoweather recorder[J]. Solid Earth Sciences, 2020, 5(4):249-253. doi: 10.1016/j.sesci.2020.10.001
[10] Yan H, Liu C C, An Z S, et al. Extreme weather events recorded by daily to hourly resolution biogeochemical proxies of marine giant clam shells[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(13):7038-7043.
[11] Lin A Y M, Meyers M A, Vecchio K S. Mechanical properties and structure of Strombus gigas, Tridacna gigas, and Haliotis rufescens sea shells: a comparative study[J]. Materials Science and Engineering: C, 2006, 26(8):1380-1389. doi: 10.1016/j.msec.2005.08.016
[12] Norton J H, Jones G W. The Giant Clam: An Anatomical and Histological Atlas[M]. Canberra, Australia: Australian Centre for International Agricultural Research, 1992.
[13] Aharon P. Recorders of reef environment histories: stable isotopes in corals, giant clams, and calcareous algae[J]. Coral Reefs, 1991, 10(2):71-90. doi: 10.1007/BF00571826
[14] Sano Y, Kobayashi S, Shirai K, et al. Past daily light cycle recorded in the strontium/calcium ratios of giant clam shells[J]. Nature Communications, 2012, 3(1):761. doi: 10.1038/ncomms1763
[15] Aharon P. 140, 000-yr isotope climatic record from raised coral reefs in New Guinea[J]. Nature, 1983, 304(5928):720-723. doi: 10.1038/304720a0
[16] Grossman E L, Ku T L. Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects[J]. Chemical Geology: Isotope Geoscience section, 1986, 59:59-74. doi: 10.1016/0168-9622(86)90057-6
[17] Watanabe T, Oba T. Daily reconstruction of water temperature from oxygen isotopic ratios of a modern Tridacna shell using a freezing microtome sampling technique[J]. Journal of Geophysical Research: Oceans, 1999, 104(C9):20667-20674. doi: 10.1029/1999JC900097
[18] Aubert A, Lazareth C E, Cabioch G, et al. The tropical giant clam Hippopus hippopus shell, a new archive of environmental conditions as revealed by sclerochronological and δ18O profiles[J]. Coral Reefs, 2009, 28(4):989-998. doi: 10.1007/s00338-009-0538-0
[19] Duprey N, Lazareth C E, Dupouy C, et al. Calibration of seawater temperature and δ 18Oseawater signals in Tridacna maxima’s δ 18Oshell record based on in situ data[J]. Coral Reefs, 2015, 34(2):437-450. doi: 10.1007/s00338-014-1245-z
[20] Wang G Z, Yan H, Liu C C, et al. Oxygen isotope temperature calibrations for modern Tridacna shells in western Pacific[J]. Coral Reefs, 2022, 41(1):113-130. doi: 10.1007/s00338-021-02208-5
[21] Pätzold J, Heinrichs J P, Wolschendorf K, et al. Correlation of stable oxygen isotope temperature record with light attenuation profiles in reef-dwelling Tridacna shells[J]. Coral Reefs, 1991, 10(2):65-69. doi: 10.1007/BF00571825
[22] Killam D, Thomas R, Al‐Najjar T, et al. Interspecific and intrashell stable isotope variation among the red sea giant clams[J]. Geochemistry, Geophysics, Geosystems, 2020, 21(7):e2019GC008669. doi: 10.1029/2019GC008669
[23] Jones D S, Williams D F, Romanek C S. Life history of symbiont-bearing giant clams from stable isotope profiles[J]. Science, 1986, 231(4733):46-48. doi: 10.1126/science.231.4733.46
[24] Yamanashi J, Takayanagi H, Isaji A, et al. Carbon and oxygen isotope records from Tridacna derasa shells: toward establishing a reliable proxy for sea surface environments[J]. PLoS One, 2016, 11(6):e0157659. doi: 10.1371/journal.pone.0157659
[25] Arias-Ruiz C, Elliot M, Bézos A, et al. Geochemical fingerprints of climate variation and the extreme La Niña 2010–11 as recorded in a Tridacna squamosa shell from Sulawesi, Indonesia[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 487:216-228. doi: 10.1016/j.palaeo.2017.08.037
[26] Yang Z K, Shao D, Mei Y J, et al. The controlling mechanism of mid- to late Holocene carbon isotopic variations of Tridacnidae in the South China Sea[J]. Marine Geology, 2019, 415:105958. doi: 10.1016/j.margeo.2019.06.003
[27] Romanek C S, Jones D S, Williams D F, et al. Stable isotopic investigation of physiological and environmental changes recorded in shell carbonate from the giant clam Tridacna maxima[J]. Marine Biology, 1987, 94(3):385-393. doi: 10.1007/BF00428244
[28] Suess H E. Natural radiocarbon and the rate of exchange of carbon dioxide be-tween the atmosphere and the sea[J]. Nuclear Processes in Geologic Settings, 1953, 43:52-56.
[29] Keeling C D. The Suess effect: 13Carbon-14Carbon interrelations[J]. Environment International, 1979, 2(4-6):229-300. doi: 10.1016/0160-4120(79)90005-9
[30] Swart P K, Greer L, Rosenheim B E, et al. The 13C Suess effect in scleractinian corals mirror changes in the anthropogenic CO2 inventory of the surface oceans[J]. Geophysical Research Letters, 2010, 37(5):L05604.
[31] Deng W F, Chen X F, Wei G J, et al. Decoupling of coral skeletal δ13C and solar irradiance over the past millennium caused by the oceanic Suess effect[J]. Paleoceanography, 2017, 32(2):161-171. doi: 10.1002/2016PA003049
[32] Dassié E P, Lemley G M, Linsley B K. The Suess effect in Fiji coral δ13C and its potential as a tracer of anthropogenic CO2 uptake[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2013, 370:30-40. doi: 10.1016/j.palaeo.2012.11.012
[33] Yan H, Soon W, Wang Y H. A composite sea surface temperature record of the northern South China Sea for the past 2500years: a unique look into seasonality and seasonal climate changes during warm and cold periods[J]. Earth-Science Reviews, 2015, 141:122-135. doi: 10.1016/j.earscirev.2014.12.003
[34] Warter V, Müller W. Daily growth and tidal rhythms in Miocene and modern giant clams revealed via ultra-high resolution LA-ICPMS analysis: a novel methodological approach towards improved sclerochemistry[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 465:362-375. doi: 10.1016/j.palaeo.2016.03.019
[35] Agbaje O B A, Thomas D E, Dominguez J G, et al. Biomacromolecules in bivalve shells with crossed lamellar architecture[J]. Journal of Materials Science, 2019, 54(6):4952-4969. doi: 10.1007/s10853-018-3165-8
[36] Zhao N Y, Yan H, Yang Y J, et al. A 23.7-year long daily growth rate record of a modern giant clam shell from South China Sea and its potential in high-resolution paleoclimate reconstruction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2021, 583:110682. doi: 10.1016/j.palaeo.2021.110682
[37] Zhao N Y, Yan H, Luo F, et al. Daily growth rate variation in Tridacna shells as a record of tropical cyclones in the South China Sea: palaeoecological implications[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2023, 615:111444. doi: 10.1016/j.palaeo.2023.111444
[38] Warter V, Erez J, Müller W. Environmental and physiological controls on daily trace element incorporation in Tridacna crocea from combined laboratory culturing and ultra-high resolution LA-ICP-MS analysis[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2018, 496:32-47. doi: 10.1016/j.palaeo.2017.12.038
[39] Aharon P, Chappell J. Oxygen isotopes, sea level changes and the temperature history of a coral reef environment in New Guinea over the last 105 years[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 1986, 56(3-4):337-379. doi: 10.1016/0031-0182(86)90101-X
[40] 洪阿实, 洪鹰, 王庆春, 等. 1994年夏季南海东北部海水氧同位素分布特征[J]. 热带海洋, 1997, 16(2):82-90 HONG Ashi, HONG Ying, WANG Qingchun, et al. Distributive characteristics of O isotope of the northeastern South China Sea in the summer of 1994[J]. Tropic Oceanology, 1997, 16(2):82-90.]
[41] Yan H, Shao D, Wang Y H, et al. Sr/Ca differences within and among three Tridacnidae species from the South China Sea: implication for paleoclimate reconstruction[J]. Chemical Geology, 2014, 390:22-31. doi: 10.1016/j.chemgeo.2014.10.011
[42] Romanek C S, Grossman E L. Stable isotope profiles of Tridacna maxima as environmental indicators[J]. Palaios, 1989, 4(5):402-413. doi: 10.2307/3514585
[43] 文汉锋, 赵楠钰, 刘成程, 等. 西太平洋帕劳砗磲高分辨率氧同位素记录及其指示的气候环境变化[J]. 海洋地质与第四纪地质, 2021, 41(1):1-13 WEN Hanfeng, ZHAO Nanyu, LIU Chengcheng, et al. High-resolution oxygen isotope records of Tridacna gigas from Palau, Western Pacific and its climatic and environmental implications[J]. Marine Geology & Quaternary Geology, 2021, 41(1):1-13.]
[44] Trofimova T, Milano S, Andersson C, et al. Oxygen isotope composition of Arctica islandica aragonite in the context of shell architectural organization: implications for paleoclimate reconstructions[J]. Geochemistry, Geophysics, Geosystems, 2018, 19(2):453-470. doi: 10.1002/2017GC007239
[45] Cusack M, Parkinson D, Freer A, et al. Oxygen isotope composition in Modiolus modiolus aragonite in the context of biological and crystallographic control[J]. Mineralogical Magazine, 2008, 72(2):569-577. doi: 10.1180/minmag.2008.072.2.569
[46] 殷建平, 王友绍, 徐继荣, 等. 海洋碳循环研究进展[J]. 生态学报, 2006, 26(2):566-575 doi: 10.3321/j.issn:1000-0933.2006.02.033 YIN Jianping, WANG Youshao, XU Jirong, et al. Adavances of studies on marine carbon cycle[J]. Acta Ecologica Sinica, 2006, 26(2):566-575.] doi: 10.3321/j.issn:1000-0933.2006.02.033
[47] McConnaughey T. 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns[J]. Geochimica et Cosmochimica Acta, 1989, 53(1):151-162. doi: 10.1016/0016-7037(89)90282-2
[48] McConnaughey T A, Burdett J, Whelan J F, et al. Carbon isotopes in biological carbonates: respiration and photosynthesis[J]. Geochimica et Cosmochimica Acta, 1997, 61(3):611-622. doi: 10.1016/S0016-7037(96)00361-4
[49] Lorrain A, Paulet Y M, Chauvaud L, et al. δ13C variation in scallop shells: increasing metabolic carbon contribution with body size?[J]. Geochimica et Cosmochimica Acta, 2004, 68(17):3509-3519. doi: 10.1016/j.gca.2004.01.025
[50] Ma X L, Yan H, Ma W T, et al. Symbiotic zooxanthellae drive the δ13C changes of Tridacna gigas shell in the southern South China Sea[J]. Journal of Geophysical Research: Biogeosciences, 2023, 128(6):e2022JG007337. doi: 10.1029/2022JG007337
[51] Klein R T, Lohmann K C, Thayer C W. SrCa and 13C12C ratios in skeletal calcite of Mytilus trossulus: covariation with metabolic rate, salinity, and carbon isotopic composition of seawater[J]. Geochimica et Cosmochimica Acta, 1996, 60(21):4207-4221. doi: 10.1016/S0016-7037(96)00232-3
[52] McConnaughey T A, Gillikin D P. Carbon isotopes in mollusk shell carbonates[J]. Geo-Marine Letters, 2008, 28(5-6):287-299. doi: 10.1007/s00367-008-0116-4
[53] McConnaughey T A. Sub-equilibrium oxygen-18 and carbon-13 levels in biological carbonates: carbonate and kinetic models[J]. Coral Reefs, 2003, 22(4):316-327. doi: 10.1007/s00338-003-0325-2
[54] Chauvaud L, Lorrain A, Dunbar R B, et al. Shell of the great scallop Pecten maximus as a high-frequency archive of paleoenvironmental changes[J]. Geochemistry, Geophysics, Geosystems, 2005, 6(8):Q08001.
[55] Grottoli A G, Wellington G M. Effect of light and zooplankton on skeletal δ13C values in the eastern Pacific corals Pavona clavus and Pavona gigantea[J]. Coral Reefs, 1999, 18(1):29-41. doi: 10.1007/s003380050150
[56] Gillikin D P, Lorrain A, Meng L, et al. A large metabolic carbon contribution to the δ13C record in marine aragonitic bivalve shells[J]. Geochimica et Cosmochimica Acta, 2007, 71(12):2936-2946. doi: 10.1016/j.gca.2007.04.003
[57] Heikoop J M, Dunn J J, Risk M J, et al. Separation of kinetic and metabolic isotope effects in carbon-13 records preserved in reef coral skeletons[J]. Geochimica et Cosmochimica Acta, 2000, 64(6):975-987. doi: 10.1016/S0016-7037(99)00363-4
[58] Soo P, Todd P A. The behaviour of giant clams (Bivalvia: Cardiidae: Tridacninae)[J]. Marine Biology, 2014, 161(12):2699-2717. doi: 10.1007/s00227-014-2545-0
[59] SWart P K. Carbon and oxygen isotope fractionation in scleractinian corals: a review[J]. Earth-Science Reviews, 1983, 19(1):51-80. doi: 10.1016/0012-8252(83)90076-4
[60] Lucas J S, Nash W J, Crawford C M, et al. Environmental influences on growth and survival during the ocean-nursery rearing of giant clams, Tridacna gigas (L. )[J]. Aquaculture, 1989, 80(1-2):45-61. doi: 10.1016/0044-8486(89)90272-X
[61] Jantzen C, Wild C, El-Zibdah M, et al. Photosynthetic performance of giant clams, Tridacna maxima and T. squamosa, Red Sea[J]. Marine Biology, 2008, 155(2):211-221. doi: 10.1007/s00227-008-1019-7
[62] Rossbach S, Saderne V, Anton A, et al. Light-dependent calcification in Red Sea giant clam Tridacna maxima[J]. Biogeosciences, 2019, 16(13):2635-2650. doi: 10.5194/bg-16-2635-2019
[63] Blidberg E, Elfwing T, Plantman P, et al. Water temperature influences on physiological behaviour in three species of giant clams (Tridacnidae)[C]//Proceedings of the 9th International Coral Reef Symposium. Bali, 2000.
[64] Elfwing T, Plantman P, Tedengren M, et al. Responses to temperature, heavy metal and sediment stress by the giant clam Tridacna squamosa[J]. Marine and Freshwater Behaviour and Physiology, 2001, 34(4):239-248. doi: 10.1080/10236240109379077
[65] Huang C Y, Wu S F, Zhao M X, et al. Surface ocean and monsoon climate variability in the South China Sea since the last glaciation[J]. Marine Micropaleontology, 1997, 32(1-2):71-94. doi: 10.1016/S0377-8398(97)00014-5
[66] Liu Y, Liu W G, Peng Z C, et al. Instability of seawater pH in the South China Sea during the mid-late Holocene: evidence from boron isotopic composition of corals[J]. Geochimica et Cosmochimica Acta, 2009, 73(5):1264-1272. doi: 10.1016/j.gca.2008.11.034
[67] Guo Y R, Deng W F, Wei G J, et al. Exploring the temperature dependence of clumped isotopes in modern Porites corals[J]. Journal of Geophysical Research: Biogeosciences, 2020, 125(1):e2019JG005402. doi: 10.1029/2019JG005402
[68] Linsley B K, Messier R G, Dunbar R B. Assessing between-colony oxygen isotope variability in the coral Porites lobata at clipperton atoll[J]. Coral Reefs, 1999, 18(1):13-27. doi: 10.1007/s003380050148
[69] Böhm F, Joachimski M M, Lehnert H, et al. Carbon isotope records from extant Caribbean and South Pacific sponges: evolution of δ13C in surface water DIC[J]. Earth and Planetary Science Letters, 1996, 139(1-2):291-303. doi: 10.1016/0012-821X(96)00006-4
[70] 陈法锦, 陈建芳, 金海燕, 等. 南海表层沉积物与沉降颗粒物中有机碳的δ13C对比研究及其古环境再造意义[J]. 沉积学报, 2012, 30(2):340-345 CHEN Fajin, CHEN Jianfang, JIN Haiyan, et al. Correlation of δ13Corg in surface sediments with sinking particulate matter in South China Sea and implication for reconstructing paleo-environment[J]. Acta Sedimentologica Sinica, 2012, 30(2):340-345.]
-
期刊类型引用(1)
1. 刘雅琦, 李建勇, 王宁练, 冉艺. 近5000 a来西天山温泉地区古火的定量重建及其驱动机制. 干旱区地理.  百度学术
百度学术
其他类型引用(0)



 下载:
下载: