Carbon cycle within the sulfate-methane transition zone in the marine sediments of Hangzhou Bay
-
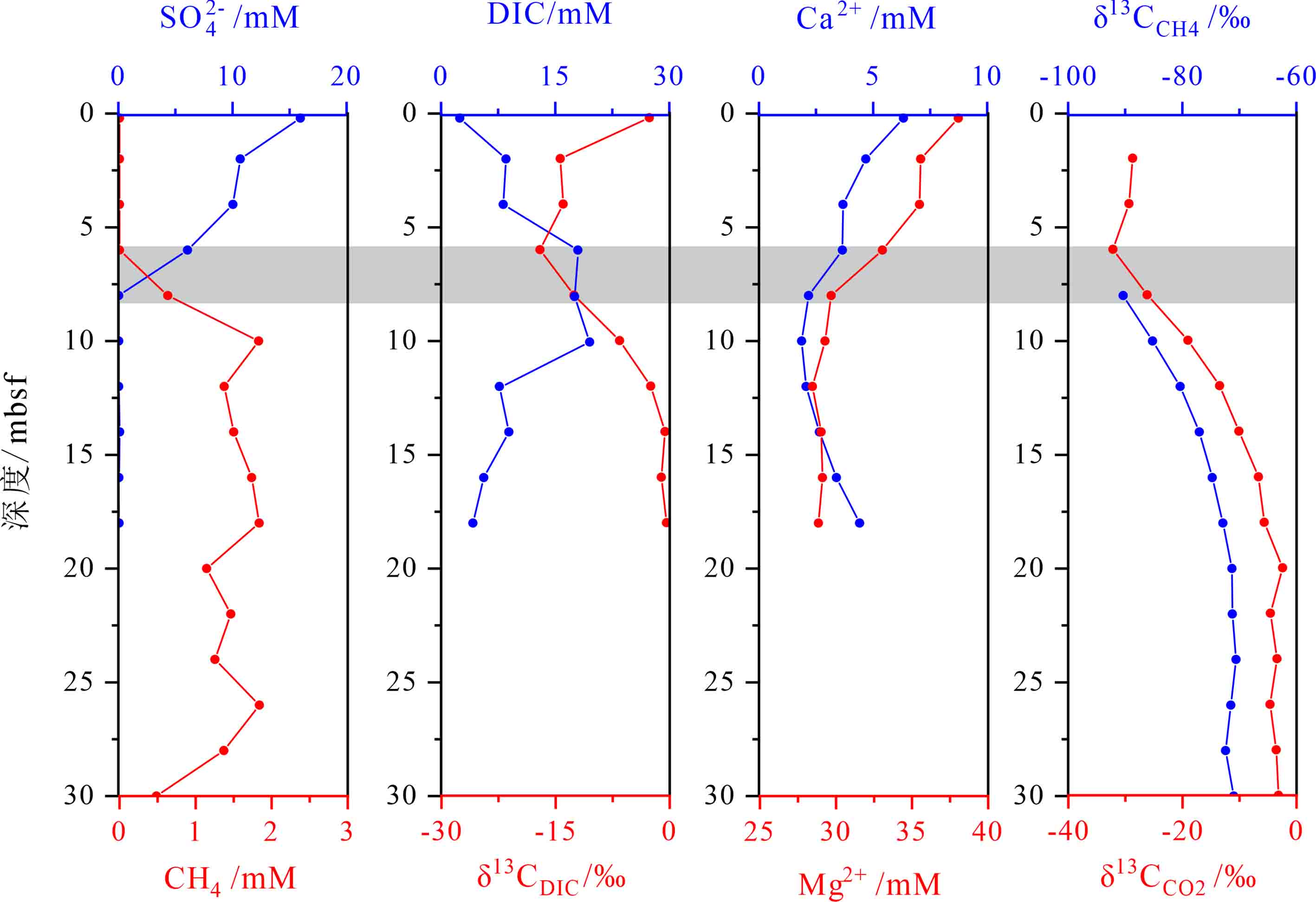
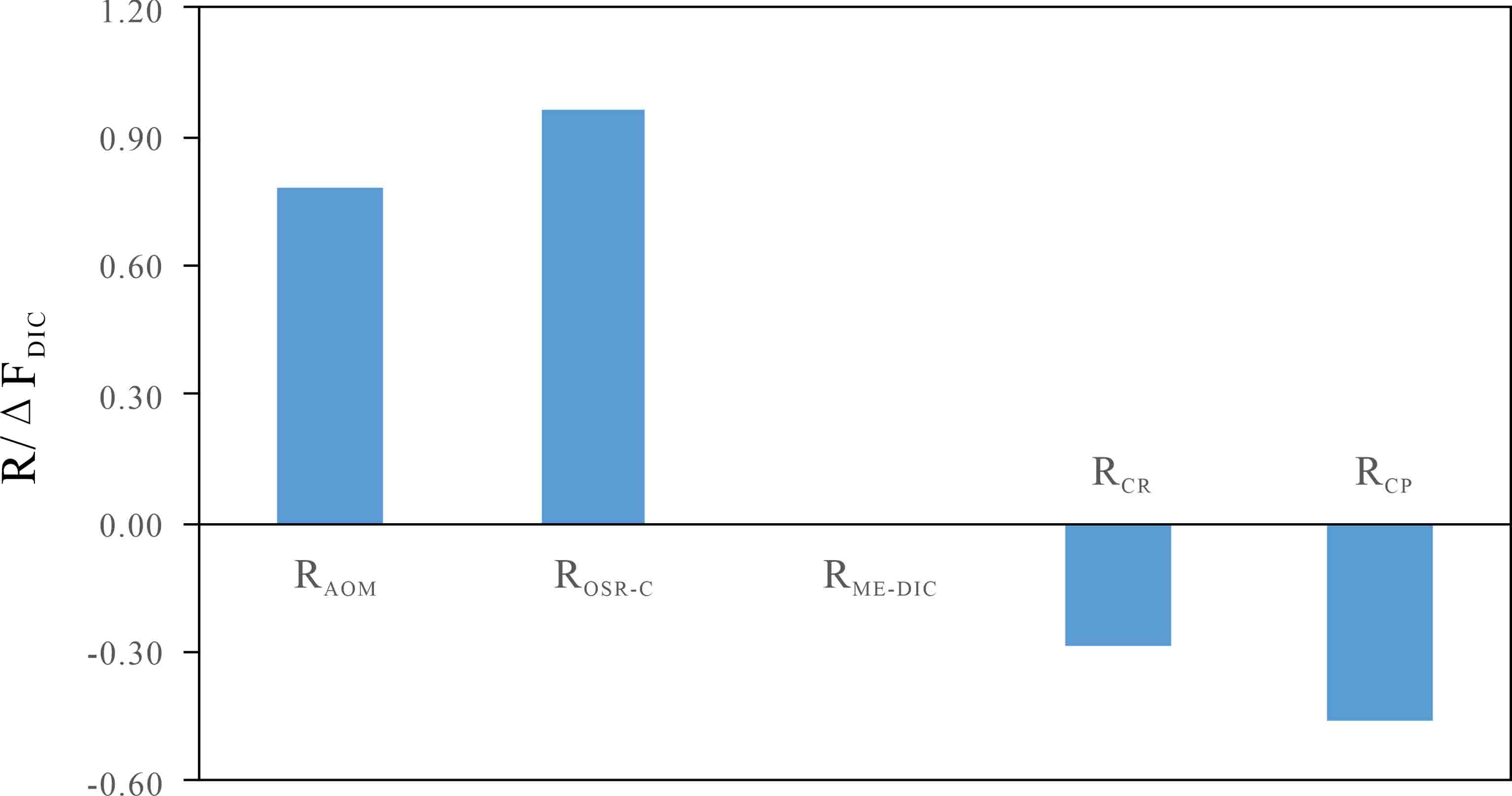
摘要: 杭州湾海底沉积物中蕴藏着大量的浅层生物气,作为温室气体CH4的重要载体,研究其甲烷厌氧氧化(AOM)及相关碳循环过程,对正确评估浅层生物气的生态环境效应具有重要的科学意义。通过对YS6孔柱状沉积物孔隙水、顶空气等地球化学参数的测试分析,基于质量平衡和碳同位素质量平衡原理,利用“箱式模型”定量研究了硫酸盐—甲烷转换带(SMTZ)内的碳循环过程。结果表明:SMTZ位于海底约6~8 mbsf沉积层,其内部碳循环过程除了包含有机质的硫酸盐还原(OSR)、AOM和碳酸盐沉淀(CP)反应外,还隐藏存在“AOM生成的溶解无机碳(DIC)”产甲烷反应(CR),反应速率分别为9.14、7.42、4.36、2.72 mmol·m−2·a−1,而有机质降解产甲烷反应(ME)未发生。各反应对SMTZ内孔隙水DIC的补充贡献率为OSR>AOM>ME,而对DIC的消耗贡献率CP>CR。深部含甲烷沉积层向上扩散而来的CH4并不是驱动SMTZ内部SO42−还原的唯一电子供体,CR和OSR反应亦是导致进入SMTZ内硫酸盐扩散通量大于甲烷的重要因素,且SMTZ下边缘沉积层出现明显的13CH4亏损亦与CR反应有关。本研究认为,定量评估海底沉积物中AOM作用的相对强弱时,SMTZ内可能存在的“隐藏的”产甲烷作用(如CR、ME等)不能忽视。
-
关键词:
- 甲烷厌氧氧化 /
- 硫酸盐—甲烷转换带内碳循环 /
- “隐藏的”产甲烷作用 /
- 箱式模型 /
- 杭州湾
Abstract: Large amount of shallow biogenic gas occurs in the marine sediments of Hangzhou Bay. As an important greenhouse gas and carbon carrier, methane and its anaerobic oxidation (AOM) and carbon cycle within the sulfate-methane transition zone (SMTZ) in marine sediments are of great significance for accurately assessment of the eco-environmental effects. Based on the test results and geochemical parameters, such as those from pore water and headspace gas in the YS6 sediment cores, following the principles of mass conservation and carbon isotope mass conservation, the internal carbon cycle in SMTZ for the YS6 was quantitatively studied with the “box model”. It is found that the SMTZ occurs in the 6~8 mbsf sediment layer.In addition to organoclastic sulfate reduction (OSR), AOM and carbonate precipitation (CP), there are concealed methanogenesis by carbon dioxide reduction of DIC produced from AOM (CR). However, methanogenesis from organic matter degradation (ME) almost not observed in the SMTZ-internal carbon cycling. The reaction rates of OSR, AOM, CP, CR and ME were 9.14 mmol·m−2·yr−1, 7.42 mmol·m−2·yr−1, 4.36 mmol·m−2·yr−1, 2.72 mmol·m−2·yr−1 and 0.00 mmol·m−2·yr−1, respectively. The contribution rate of each reaction to pore water DIC in SMTZ was in an order of OSR>AOM>ME (ME= 0), while the consumption rate was CP>CR. Methane diffused upward from deeper methane zone was not the only electron donor to drive the internal sulfate reduction (SR) in SMTZ. CR and OSR were also the important factors for sulfate flux into SMTZ to be greater than methan , and the obvious 13C-depletion of methane in the lower border of SMTZ was also related to the CR. When quantitatively evaluating the relative strength of AOM in marine sediments, the “cryptic” methanogenesis (such as CR, ME, etc.) in SMTZ cannot be ignored.-
Keywords:
- AOM /
- SMTZ-internal carbon cycling /
- cryptic methanogenesis /
- box model /
- Hangzhou Bay
-
-
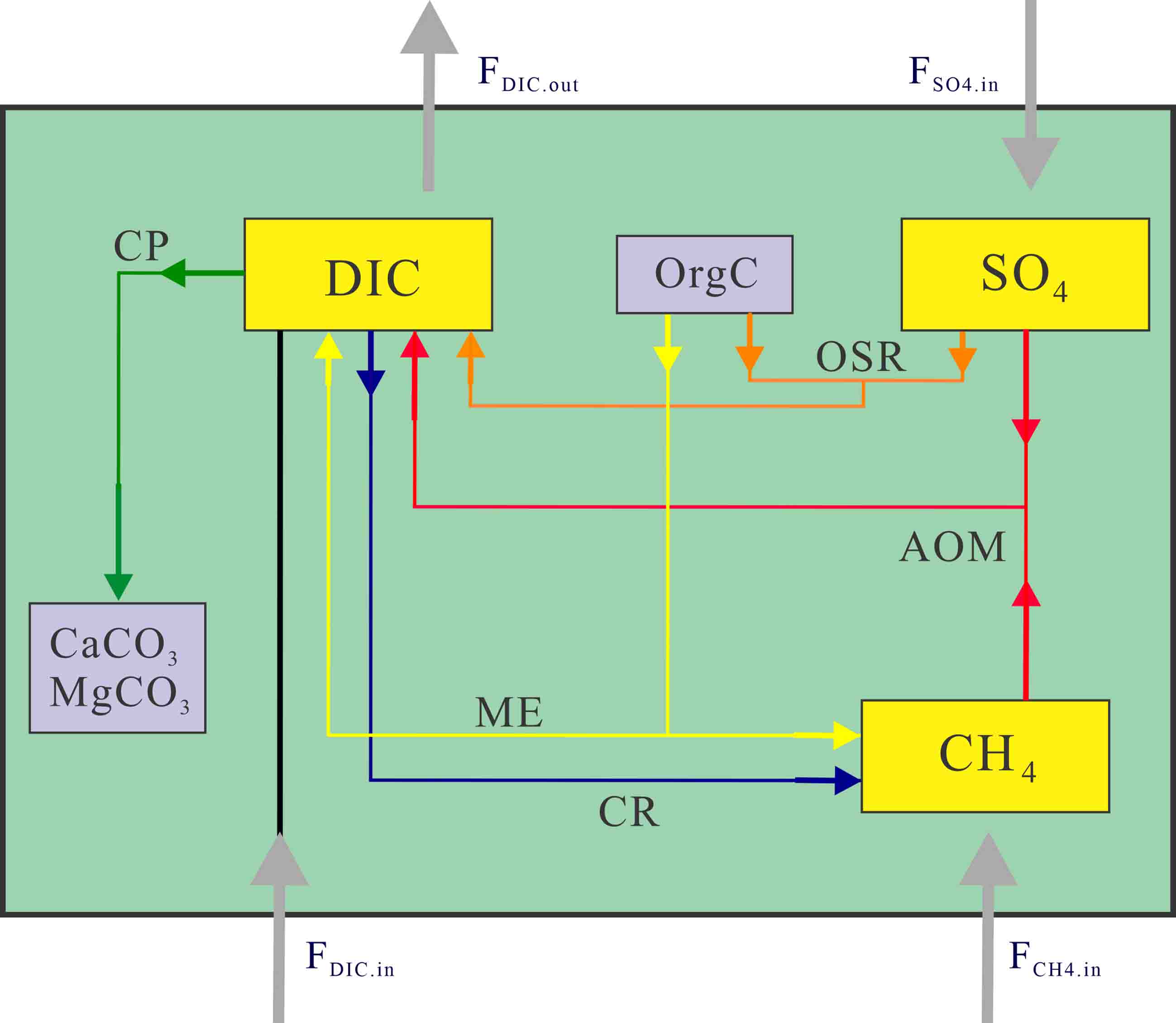
图 2 SMTZ内碳循环“箱式模型”图[14]
溶解组分用黄色框表示,固体组分用灰色框表示;灰线箭头表示模型域之外的DIC、SO42−和CH4的输入/输出;5个主要反应路径AOM、OSR、CR、ME和CP分别用不同颜色的箭头标识。
Figure 2. Illustration of box model for SMTZ-internal carbon cycling[14]
The dissolved components are shown in yellow and the solid components in gray; The gray arrow represents the input/output of DIC, SO42− and CH4 outside the box model; The reaction paths of AOM, OSR, CR, ME and CP are marked by different colored arrows.
表 1 孔隙水部分溶解组分的扩散通量
Table 1 Diffusion fluxes of dissolved components in pore water
组分 扩散系数D0/(m2·s−1) 扩散通量F/(mmol·m−2·a−1) 符号 SO42− 8.91E-10 11.87 FSO4.in CH4 1.39E-09 4.65 FCH4.in Ca2+ 6.72E-10 2.29 FCa.in 0.76 FCa.out Mg2+ 5.91E-10 4.15 FMg.in 1.33 FMg.out DIC 9.89E-10 16.72 FDIC.out 7.23 FDIC.in 表 2 SMTZ内轻、重碳同位素反应速率和总反应速率
Table 2 The reaction rates of light and heavy carbon isotopes in SMTZ and the total reaction rates
项目 参数 计算结果 轻、重DIC通量(mmol·m−2·a−1) 12FDIC.out −16.53 12FDIC.in −7.15 13FDIC.out −0.18 13FDIC.in −0.08 碳同位素值(‰) δ13COM −23.32 δ13CCH4-SMTZ −90.34 δ13CDIC-SMTZ −17.04 δ13CCH4-bottom −71.31 δ13CDIC-bottom −0.42 13C/12C比值 rstd 0.011 237 rOM 0.010 975 rCH4-SMTZ 0.010 222 rDIC-SMTZ 0.011 046 rCH4-bottom 0.010 436 rDIC-bottom 0.011 232 分馏系数 αCR 1.070 9 αAOM 1.017 0 反应分数 f 0.00 0.50 1.00 b 0.37 0.37 0.37 轻同位素反应速率
(mmol·m−2·a−1)12RAOM 11.87 9.26 7.35 12RCR 4.35 3.39 2.69 12RCP 4.31 4.31 4.31 12ROM 12.34 10.44 9.04 12RME 12.34 5.22 0.00 12RME-CH4 6.17 2.61 0.00 12RME-DIC 6.17 2.61 0.00 12ROSR-C 0.00 5.22 9.04 重同位素反应速率
(mmol·m−2·a−1)13RAOM 0.12 0.09 0.07 13RCR 0.04 0.03 0.03 13RCP 0.05 0.05 0.05 13ROM 0.14 0.11 0.10 13RME 0.14 0.06 0.00 13RME-CH4 0.06 0.03 0.00 13RME-DIC 0.07 0.03 0.00 13ROSR-C 0.00 0.06 0.10 总反应速率
(mmol·m−2·a−1)RAOM 11.99 9.35 7.42 RCR 4.39 3.42 2.72 RCP 4.36 4.36 4.36 ROM 12.48 10.55 9.14 RME 12.48 5.28 0.00 RME-CH4 6.24 2.64 0.00 RME-DIC 6.24 2.64 0.00 ROSR-C 0.00 5.28 9.14 甲烷通量绝对差值(mmol·m−2·a−1) △FCH4 3.29 1.36 0.06 注:△FCH4= FCH4.in−(RAOM−RCR−RME-CH4)。 -
[1] Milkov A V. Global estimates of hydrate-bound gas in marine sediments: how much is really out there? [J]. Earth-Science Reviews, 2004, 66(3-4): 183-197. doi: 10.1016/j.earscirev.2003.11.002
[2] Reeburgh W S. Oceanic methane biogeochemistry [J]. Chemical Reviews, 2007, 107(2): 486-513. doi: 10.1021/cr050362v
[3] Regnier P, Dale A W, Arndt S, et al. Quantitative analysis of anaerobic oxidation of methane (AOM) in marine sediments: a modeling perspective [J]. Earth-Science Reviews, 2011, 106(1-2): 105-130. doi: 10.1016/j.earscirev.2011.01.002
[4] Blair N E, Aller R C. Anaerobic methane oxidation on the Amazon shelf [J]. Geochimica et Cosmochimica Acta, 1995, 59(18): 3707-3715. doi: 10.1016/0016-7037(95)00277-7
[5] Borowski W S, Paull C K, Ussler III W. Marine pore-water sulfate profiles indicate in situ methane flux from underlying gas hydrate [J]. Geology, 1996, 24(7): 655-658. doi: 10.1130/0091-7613(1996)024<0655:MPWSPI>2.3.CO;2
[6] Egger M, Riedinger N, Mogollón J M, et al. Global diffusive fluxes of methane in marine sediments [J]. Nature Geoscience, 2018, 11(6): 421-245. doi: 10.1038/s41561-018-0122-8
[7] Beulig F, Røy H, McGlynn S E, et al. Cryptic CH4 cycling in the sulfate–methane transition of marine sediments apparently mediated by ANME-1 archaea [J]. ISME J, 2018, 13(2): 250-262.
[8] Flury S, Røy H, Dale A W, et al. Controls on subsurface methane fluxes and shallow gas formation in Baltic Sea sediment (Aarhus Bay, Denmark) [J]. Geochimica et Cosmochimica Acta, 2016, 188: 297-309. doi: 10.1016/j.gca.2016.05.037
[9] Komada T, Burdige D J, Li H L, et al. Organic matter cycling across the sulfate-methane transition zone of the Santa Barbara Basin, California Borderland [J]. Geochimica et Cosmochimica Acta, 2016, 176: 259-278. doi: 10.1016/j.gca.2015.12.022
[10] Yoshinaga M Y, Holler T, Goldhammer T, et al. Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane [J]. Nature Geoscience, 2014, 7(3): 190-194. doi: 10.1038/ngeo2069
[11] Kim S, Choi K, Chung J. Reduction in carbon dioxide and production of methane by biological reaction in the electronics industry [J]. International Journal of Hydrogen Energy, 2013, 38(8): 3488-3496. doi: 10.1016/j.ijhydene.2012.12.007
[12] Lash G G. Significance of stable carbon isotope trends in carbonate concretions formed in association with anaerobic oxidation of methane (AOM), Middle and Upper Devonian shale succession, western New York State, USA [J]. Marine and Petroleum Geology, 2018, 91: 470-479. doi: 10.1016/j.marpetgeo.2018.01.032
[13] Chuang P C, Frank Y T, Wallmann K, et al. Carbon isotope exchange during Anaerobic Oxidation of Methane (AOM) in sediments of the northeastern South China Sea [J]. Geochimica et Cosmochimica Acta, 2019, 246: 138-155. doi: 10.1016/j.gca.2018.11.003
[14] Hong W L, Torres M E, Kim J H, et al. Carbon cycling within the sulfate-methane-transition-zone in marine sediments from the Ulleung Basin [J]. Biogeochemistry, 2013, 115(1-3): 129-148. doi: 10.1007/s10533-012-9824-y
[15] Ni Y Y, Dai J X, Zou C N, et al. Geochemical characteristics of biogenic gases in China [J]. International Journal of Coal Geology, 2013, 113: 76-87. doi: 10.1016/j.coal.2012.07.003
[16] 柴小平, 胡宝兰, 魏娜, 等. 杭州湾及邻近海域表层沉积物重金属的分布、来源及评价[J]. 环境科学学报, 2015, 35(12):3906-3916. [CHAI Xiaoping, HU Baolan, WEI Na, et al. Distribution, sources and assessment of heavy metals in surface sediments of the Hangzhou Bay and its adjacent areas [J]. Acta Scientiae Circumstantiae, 2015, 35(12): 3906-3916. [17] 夏小明, 杨辉, 李炎, 等. 长江口-杭州湾毗连海区的现代沉积速率[J]. 沉积学报, 2004, 22(1):130-135. [XIA Xiaming, YANG Hui, LI Yan, et al. Modern sedimentation rates in the contiguous sea area of Changjiang Estuary and Hangzhou Bay [J]. Acta Sedimentologica Sinica, 2004, 22(1): 130-135. doi: 10.3969/j.issn.1000-0550.2004.01.020 [18] Xu F L, Ji Z Q, Wang K, et al. The distribution of sedimentary organic matter and implication of its transfer from Changjiang Estuary to Hangzhou Bay, China [J]. Open Journal of Marine Science, 2016, 6(1): 103-114. doi: 10.4236/ojms.2016.61010
[19] 陈少平, 孙家振, 沈传波, 等. 杭州湾地区浅层气成藏条件分析[J]. 华东地质学院学报, 2003, 26(4):352-356. [CHEN Shaoping, SUN Jiazhen, SHEN Chuanbo, et al. Reservoir formation condition of shallow gas in the area of Hangzhou Bay [J]. Journal of East China Geological Institute, 2003, 26(4): 352-356. [20] 胡新强, 顾兆峰, 张训华, 等. 长江口外海域浅层气地震反射形态特征及分布[J]. 海洋地质与第四纪地质, 2016, 36(1):151-157. [HU Xinqiang, GU Zhaofeng, ZHANG Xunhua, et al. Seismic shape features and distribution of shallow gas in the sea area off the Yangtze River Estuary [J]. Marine Geology & Quaternary Geology, 2016, 36(1): 151-157. [21] 杨涛, 蒋少涌, 赖鸣远, 等. 海洋沉积物孔隙水中溶解无机碳(DIC)的碳同位素分析方法[J]. 地球学报, 2005, 26(S1):51-52. [YANG Tao, JIANG Shaoyong, LAI Mingyuan, et al. An analytical method for carbon isotopic composition of dissolved inorganic carbon (DIC) in pore waters from marine sediments [J]. Acta Geoscientica Sinica, 2005, 26(S1): 51-52. [22] 杨涛, 蒋少涌, 赖鸣远, 等. 连续流同位素质谱测定水中溶解无机碳含量和碳同位素组成的方法研究[J]. 地球化学, 2006, 35(6):675-680. [YANG Tao, JIANG Shaoyong, LAI Mingyuan, et al. Analytical method for concentration and carbon isotopic composition of dissolved inorganic carbon (DIC) by continuous flow-isotope ratio mass spectrometer [J]. Geochimica, 2006, 35(6): 675-680. doi: 10.3321/j.issn:0379-1726.2006.06.014 [23] 张媛媛, 林学辉, 贺行良, 等. 离子色谱法同时测定海洋沉积物中氯和硫[J]. 分析科学学报, 2015, 31(2):249-252. [ZHANG Yuanyuan, LIN Xuehui, HE Xingliang, et al. Determination of chlorine and sulfur in marine sediment by ion chromatography [J]. Journal of Analytical Science, 2015, 31(2): 249-252. [24] Snyder G T, Hiruta A, Matsumoto R, et al. Pore water profiles and authigenic mineralization in shallow marine sediments above the methane-charged system on Umitaka Spur, Japan Sea [J]. Deep Sea Research Part II: Topical Studies in Oceanography, 2007, 54(11-13): 1216-1239. doi: 10.1016/j.dsr2.2007.04.001
[25] 贺行良, 夏宁, 刘昌岭, 等. FID/TCD并联气相色谱法测定天然气水合物的气体组成[J]. 分析测试学报, 2012, 31(2):206-210. [HE Xingliang, XIA Ning, LIU Changling, et al. Compositional analysis of gases in natural gas hydrates by GC-FID/TCD [J]. Journal of Instrumental Analysis, 2012, 31(2): 206-210. doi: 10.3969/j.issn.1004-4957.2012.02.017 [26] 贺行良, 刘昌岭, 王江涛, 等. 气相色谱-同位素比值质谱法测定天然气水合物气体单体碳氢同位素[J]. 岩矿测试, 2012, 31(1):154-158. [HE Xingliang, LIU Changling, WANG Jiangtao, et al. Measurement of carbon and hydrogen isotopes of natural gas hydrate-bound gases by gas chromatography-isotope ratio mass spectrometry [J]. Rock and Mineral Analysis, 2012, 31(1): 154-158. doi: 10.3969/j.issn.0254-5357.2012.01.021 [27] Iversen N, Jørgensen B B. Diffusion coefficients of sulfate and methane in marine sediments: Influence of porosity [J]. Geochimica et Cosmochimica Acta, 1993, 57(3): 571-578. doi: 10.1016/0016-7037(93)90368-7
[28] Boudreau B P. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments[M]. Berlin: Springer, 1997.
[29] Schulz H D. Quantification of early diagenesis: dissolved constituents in pore water and signals in the solid phase[M]//Schulz H D, Zabel M. Marine Geochemistry. Berlin, Heidelberg: Springer, 2006: 73-124.
[30] Wehrmann L M, Risgaard-Petersen N, Schrum H N, et al. Coupled organic and inorganic carbon cycling in the deep subseafloor sediment of the northeastern Bering Sea Slope (IODP Exp. 323) [J]. Chemical Geology, 2011, 284(3-4): 251-261. doi: 10.1016/j.chemgeo.2011.03.002
[31] Hu C Y, Yang T F, Burr G S, et al. Biogeochemical cycles at the sulfate-methane transition zone (SMTZ) and geochemical characteristics of the pore fluids offshore southwestern Taiwan [J]. Journal of Asian Earth Sciences, 2017, 149: 172-183. doi: 10.1016/j.jseaes.2017.07.002
[32] Rees C E. A steady-state model for sulphur isotope fractionation in bacterial reduction processes [J]. Geochimica et Cosmochimica Acta, 1973, 37(5): 1141-1162. doi: 10.1016/0016-7037(73)90052-5
[33] Bayon G, Pierre C, Etoubleau J, et al. Sr/Ca and Mg/Ca ratios in Niger Delta sediments: Implications for authigenic carbonate genesis in cold seep environments [J]. Marine Geology, 2007, 241(1-4): 93-109. doi: 10.1016/j.margeo.2007.03.007
[34] Nöthen K, Kasten S. Reconstructing changes in seep activity by means of pore water and solid phase Sr/Ca and Mg/Ca ratios in pockmark sediments of the Northern Congo Fan [J]. Marine Geology, 2011, 287(1-4): 1-13. doi: 10.1016/j.margeo.2011.06.008
[35] Whiticar M J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane [J]. Chemical Geology, 1999, 161(1-3): 291-314. doi: 10.1016/S0009-2541(99)00092-3
[36] Treude T, Krause S, Maltby J, et al. Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implications for deep sulfur cycling [J]. Geochimica et Cosmochimica Acta, 2014, 144: 217-237. doi: 10.1016/j.gca.2014.08.018
[37] Borowski W S, Paull C K, Ussler III W. Carbon cycling within the upper methanogenic zone of continental rise sediments; an example from the methane-rich sediments overlying the Blake Ridge gas hydrate deposits [J]. Marine Chemistry, 1997, 57(3-4): 299-311. doi: 10.1016/S0304-4203(97)00019-4
[38] Mazumdar A, Peketi A, Joao H M, et al. Pore-water chemistry of sediment cores off Mahanadi Basin, Bay of Bengal: Possible link to deep seated methane hydrate deposit [J]. Marine and Petroleum Geology, 2014, 49: 162-175. doi: 10.1016/j.marpetgeo.2013.10.011
[39] Wallace P J, Dickens G R, Paull C K, et al. Effects of core retrieval and degassing on the carbon isotope composition of methane in gas hydrate-and free gas-bearing sediments from the Blake Ridge[C]//Proceedings of the Ocean Drilling Program. Scientific Results. College Station, TX: Texas A&M University, 2000, 164: 101-112.



 下载:
下载: